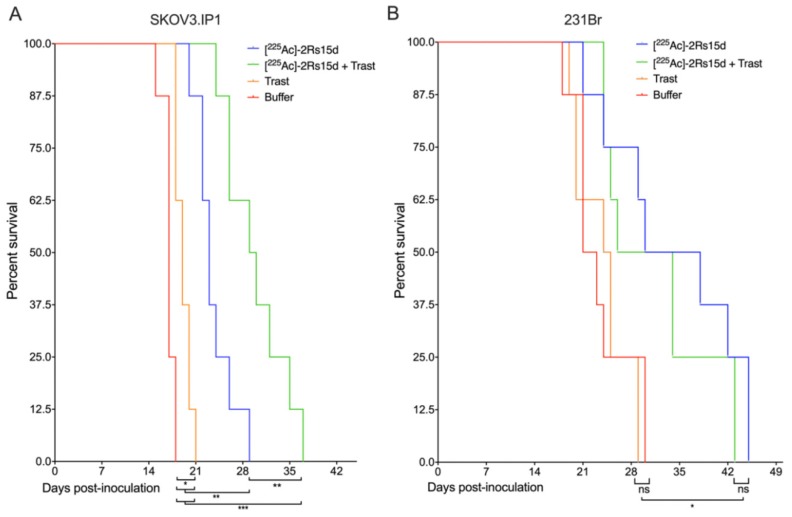
Figure 6.
Event-free survival during targeted alpha therapy. Events were defined as (i) mortality, (ii) weight loss > 20%, (iii) immobility, (iv) unresponsiveness to external stimuli. Trastuzumab-sensitive SKOV3.IP1 (A) and trastuzumab-resistant 231Br (B) tumor-bearing mice (n = 8 per group, tumor inoculation on Day 0) were treated with intravenous injections of either [225Ac]-2Rs15d (Day 7, 10, 14), combination treatment of [225Ac]-2Rs15d (Day 7, 14, 21) and trastuzumab (Loading dose; day 7-maintenance dose; day 10, 14, 17, 21, 24, 28) or trastuzumab as single agent (Loading dose; day 7-maintenance dose; day 10, 14, 17, 21, 24, 28). The control group received vehicle buffer at identical timepoints as treated groups. 150 mg/kg gelofusin was co-administered with [225Ac]-2Rs15d in order to reduce kidney retention. (ns: not significant, * p < 0.05, ** p < 0.01, *** p < 0.005).