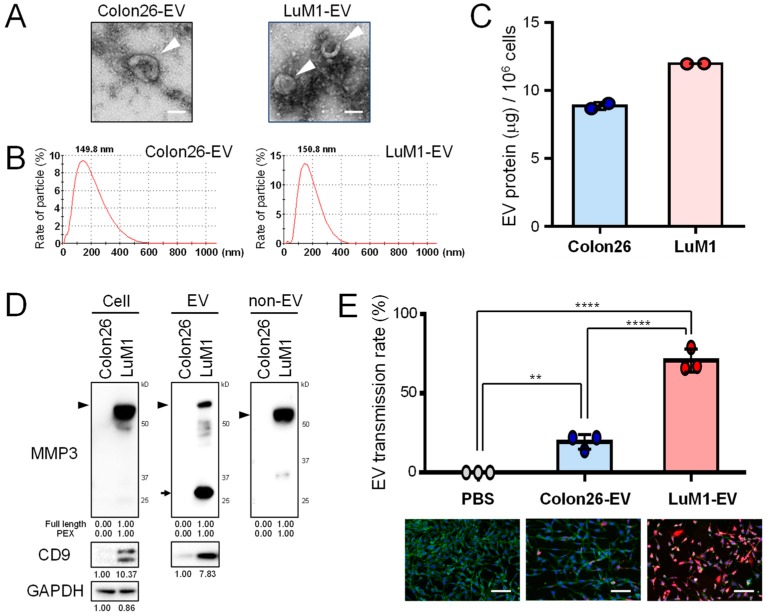
Figure 1.
Metastatic tumor-derived, matrix metalloproteinase 3 (MMP3)-rich extracellular vesicles (EVs) are highly transmissive. The roles of EVs derived from low-metastatic Colon26 cells vs. high-metastatic LuM1 cells were compared. (A) Representative TEM images of EVs. Arrowheads indicate EVs with cup-shaped morphology. Scale bars, 100 nm. (B) Particle diameter distribution of EV fractions. (C) EV protein release from Colon26 vs LuM1 cells. EV protein concentrations (μg per 106 cells) were shown. (D) Western blot analysis of MMP3 and CD9 in cell lysates, EV and non-EV fractions. Arrowhead indicates full-length MMP3 (54 kD). Arrows indicate the 25-kD PEX isoform of MMP3. For MMP3, the protein amount equivalent to 8 × 104 cells (Colon26, 0.72 μg; LuM1, 0.96 μg) was loaded to each lane. For CD9 and GAPDH, the protein amount equivalent to 1.2 × 105 cells (Colon26, 1.09 μg; LuM1, 1.44 μg) was loaded to each lane. (E) Transmission efficiencies of Colon26-EVs vs LuM1-EVs. EVs were labeled with red fluorescent ceramide and added to culture media of recipient Colon26 cells at a final concentration of 11.5 μg/mL for 24 h. Cells were fixed and stained with ActinGreen and DAPI. Top, EV transmission efficiencies. The efficiencies were the ratio of transmitted EV-positive cells to the total number of cells. ** p < 0.01, **** p < 0.0001, n = 3 fields. Bottom, representative images of EV transmission. Scale bars, 100 μm. The experiments were repeated twice in Figure 1C–E.