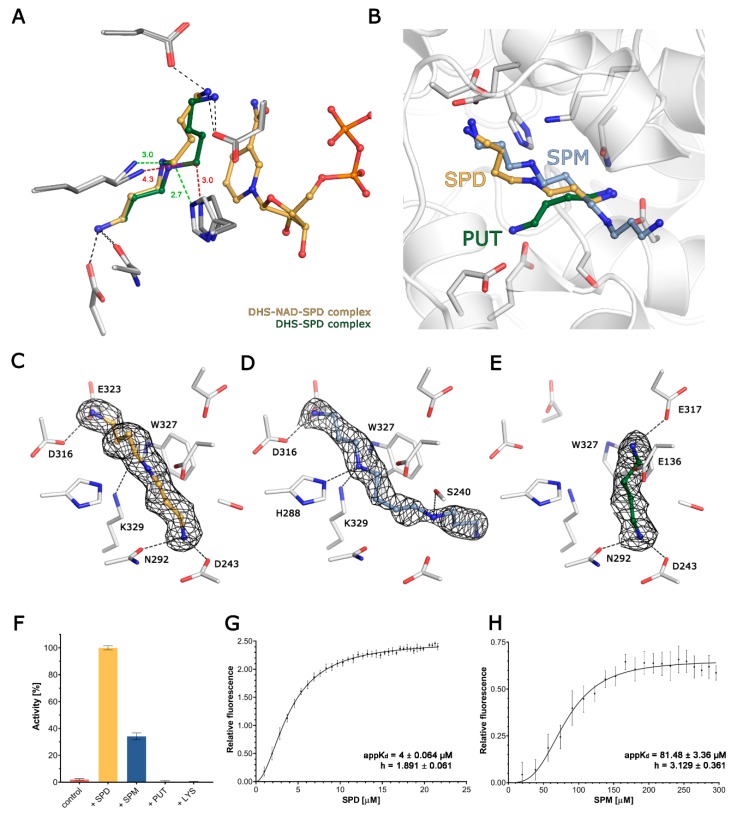
Figure 2.
The binding of polyamines to DHS. (A) The superposition of binary DHS-SPD and ternary DHS-NAD-SPD complexes. The ligands are shown as balls and sticks, with carbons coloured green in binary complexes and yellow in ternary complexes respectively. The side chains for the residues coordinating SPD are shown as sticks, in light grey for DHS-NAD-SPD and in dark grey for DHS-SPD. The change in conformation of SPD induced by the presence of NAD is highlighted by a change of distances between C5 and His288 and the catalytical lysine 329, coloured red and green for binary and ternary complexes respectively. (B) Superposition of the DHS-SPD, DHS-SPM and DHS-PUT complex structures shows the relative positions of the investigated polyamines in the active site. Ligands are represented as balls and sticks, and the residues forming binding sites are shown as sticks. (C–E) The binding of SPD, SPM and PUT is shown with ligand as balls and sticks. 2Fo-Fc composite omit maps countered at 1σ are shown as a black mesh for each ligand. Residues taking part in the binding of each ligand are labelled. (F) The relative activity of DHS, expressed as an increase in the NAD fluorescence in the presence of various ligands. (G,H) A FRET binding assay for SPD and SPM.