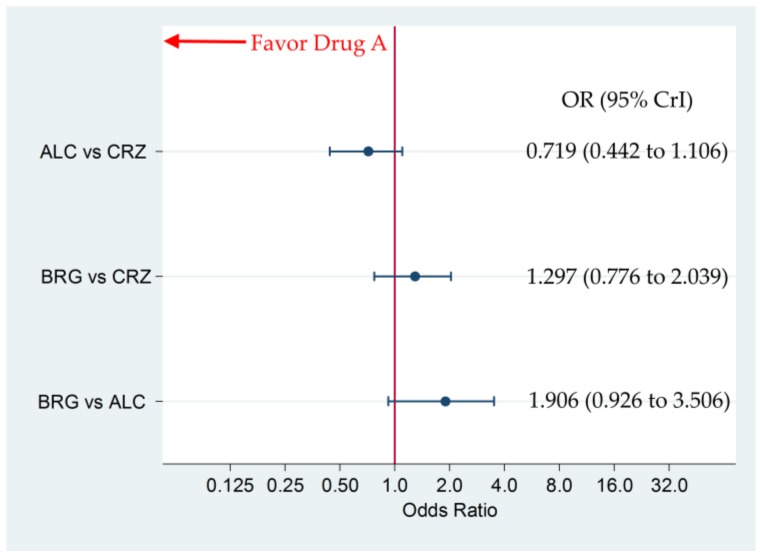
Figure 5.
The comparative safety, in terms of “any adverse events” of grades 3–5 (G3–5AAEs), of brigatinib 180 mg once daily (7-day run-in period of 90 mg once daily) and alectinib 300/600 mg twice daily in all participants. The comparisons are expressed as drug A versus drug B. Data are expressed as odds ratio (OR) and 95% credible intervals (CrIs); ALC, alectinib; CRZ, crizotinib; BRG, brigatinib.