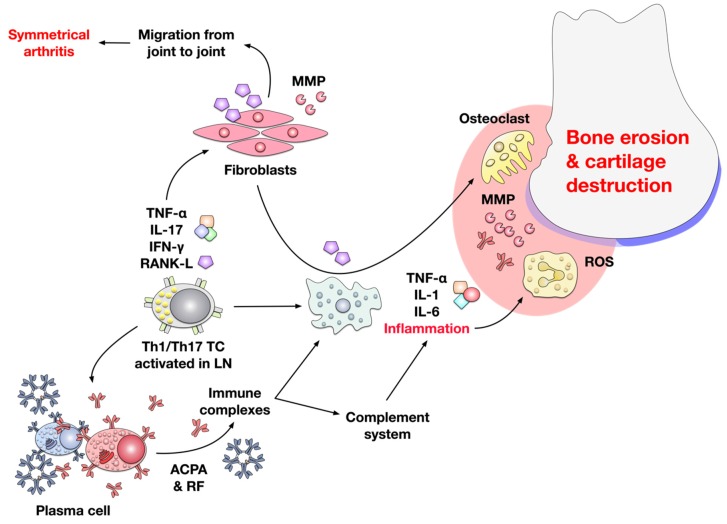
Figure 2.
Pathomechanism of RA. Inflammation in RA is induced by autoreactive Th1- or Th17 T cells primed in the lymph nodes (LN) or locally by activated Antigen-presenting cells (APCs) that present autoantigen-derived peptides. In the affected joint, activated autoreactive T cells subsequently activate macrophages and fibroblasts via the secretion of the pro-inflammatory mediators TNF-α, IL-17, IFN-γ, and receptor activator of nuclear factor KB ligand (RANK-L). Activated macrophages in turn secrete large amounts of the strongly pro-inflammatory cytokines TNF-α, IL-1β, and IL-6 which promote the establishment and maintenance of an inflammatory milieu in the synovium. Activated T cells also provide help to autoreactive B cells resulting in the production of anti-citrullinated protein antibodies (ACPAs) and rheumatoid factor (RF) autoantibodies. These autoantibodies further drive inflammation by either direct macrophage activation of triggering the complement cascade. In addition, RANK-L produced by the activated fibroblasts promotes the differentiation of osteoclasts from macrophages. Together with fibroblast-derived matrix metalloproteases (MMPs), osteoclasts, and antibodies, activated neutrophils mediate inflammation-dependent cartilage destruction and bone erosion.