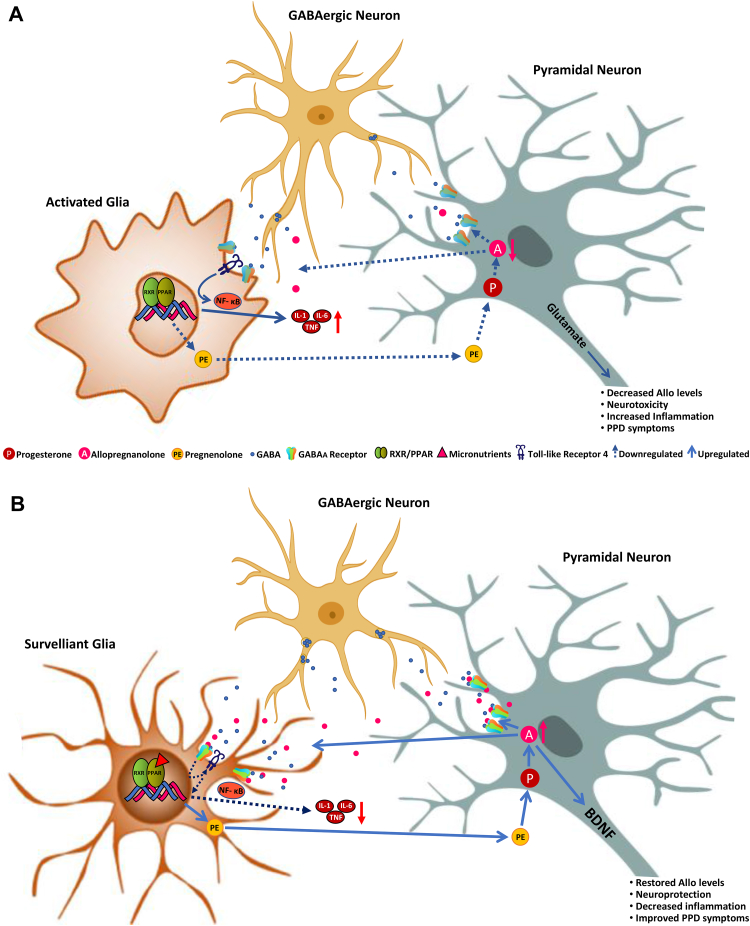
Fig. 1.
A. Schematic representation of the relationship between the GABAergic neurosteroid, allopregnanolone, microglial activation and PPAR-α stimulation in postpartum depression (PPD). Immediately after delivery and during the first weeks of the postpartum period, the dramatic drop in circulating progesterone leads to decreased levels of allopregnanolone, which is synthesized from peripherally-derived progesterone by glutamatergic neurons, including pyramidal neurons of the frontal cortex, granular cells of the dentate gyrus and CA1-3 pyramidal neurons in the hippocampus, and pyramidal-like neurons of the basolateral amygdala both in rodent and human brain (Agís-Balboa et al., 2006, Agís-Balboa et al., 2014; reviewed in Pinna et al., 2008). Allopregnanolone plays a central neuromodulatory role in facilitating the action of GABA at GABAA receptors (Majewska et al., 1986) and endogenously produced allopregnanolone plays a neurophysiological role in the fine-tuning of the GABAA receptors to GABAmimetics, positive allosteric modulators, and GABA agonists (Pinna et al., 2000). By this mechanism, allopregnanolone also regulates emotional behavior and stress-responses. Prolonged stress in animal models results in decreased corticolimbic allopregnanolone levels, which is associated with behavioral dysfunction, including elevated aggressiveness, anxiety-like and depressive-like behavior, and exaggerated fear responses and impaired contextual fear extinction (Pinna et al., 2003, 2008; Pibiri et al., 2008; Locci and Pinna, 2017, 2019). Low levels of allopregnanolone and symptoms of depression and PTSD have been observed in several clinical studies (Uzunova et al., 1998; Romeo et al., 1998; Rasmusson et al., 2006, 2019; Agis-Balboa et al., 2014; Kim et al., 2020). These neurosteroid deficits result in alterations of GABA and glutamate neurotransmission and in changes in GABAA receptor sensitivity (reviewed by Pinna, 2018) causing a GABAergic/glutamatergic imbalance. Moreover, decreased allopregnanolone levels in the postpartum period is associated with increased inflammation likely by activated microglia, which releases pro-inflammatory biomarkers, such as IL-1, IL-6 and TNF-α via the NFκB pathway that is also regulated by PPAR. Another mechanism involves the toll-like receptor 4 (TLR4), which, once activated by different triggers such as lipopolysaccharide (LPS), pathogen-associated molecular patterns (PAMPs), alcohol, stress or decreased levels of pregnenolone, forms a complex with intracellular co-activators, such as TIR Domain-Containing Adaptor Protein (TIRAP) and TRIF-related Adaptor Molecule (TRAM) to initiate a pro-inflammatory cascade that leads to NFκB activation and pro-inflammatory cytokines release (Li et al., 2016). Low levels of allopregnanolone lead to increased calcium channel activity in activated nerve terminals and increased release of glutamate that facilitates excitotoxicity mechanisms (Hu et al., 2007). Unhealthy diets, including high fatty diets or alcohol abuse play deleterious effects on PPAR function that fails to regulate pro-inflammatory processes and greatly contribute to the neuroinflammation mechanisms underlying the pathogenesis of major depression and, possibly, PPD (Henriques et al., 2018; Orio et al., 2019). Furthermore, neuroinflammation-associated release of glutamate from activated microglia worsens the neurodegenerative process found in mood disorders. B. A schematic representation of the regulatory effects of PPARs following its activation by micronutrients found in functional food that show the ability to bind to PPAR-α. PPAR-α activation by its endogenous modulator, PEA results in enhancement of allopregnanolone biosynthesis, by upregulating the expression of neurosteroidogenic enzymes and proteins, including StAR, P450ssc, that facilitate the conversion of cholesterol into pregnenolone, the precursor of all neurosteroids. Pregnenolone is then taken up by glutamatergic neurons and further converted into progesterone and allopregnanolone by the rate-limiting step enzymes, 5α-reductase type I (5α-RI) and 3α-hydroxysteroid dehydrogenase (3α-HSD) in several corticolimbic areas (Locci and Pinna, 2019). In this scenario, restored allopregnanolone binding at GABAA receptors that are expressed in microglia (Agís-Balboa et al., 2007, Agís-Balboa et al., 2006; Lee et al., 2011), dampens inflammatory processes by an effect mediated through inhibition of TLR4 that results in the subsequent repression of NFκB signaling cascade (Singh et al., 2012; Lee et al., 2011; Noorbakhsh et al., 2014). Altogether, these actions lead to downregulation in the release of pro-inflammatory cytokines, such as TNF-α and IL-6 and attenuate neurotoxicity. Similar results are observed by administering a diet with functional foods rich in bioactive micronutrients, such as fatty acids, flavonoids, minerals, and vitamins, which represent a non-pharmacological strategy to potentiate PPAR expression and function. PPAR-activation in glia engages neuronal allopregnanolone biosynthesis to regulate inhibition of inflammatory mechanisms, which are ultimately mediated by potentiation of glia GABAergic neurotransmission. At the same time, increased allopregnanolone levels in neurons may stimulate brain derived neurotropic factor (BDNF) and exert important neuroprotective functions (Nin et al., 2011; Almeida et al., 2019). We suggest that healthy diets enriched in micronutrients that are PPAR-agonists by enhancing the PPAR-allopregnanolone axis and decreasing inflammation may offer an alternative strategy to pharmacological treatments to prevent and safely treat mood disorders, including PPD.