Figure 5. Smart immunomodulation involving cancer nanomedicine.
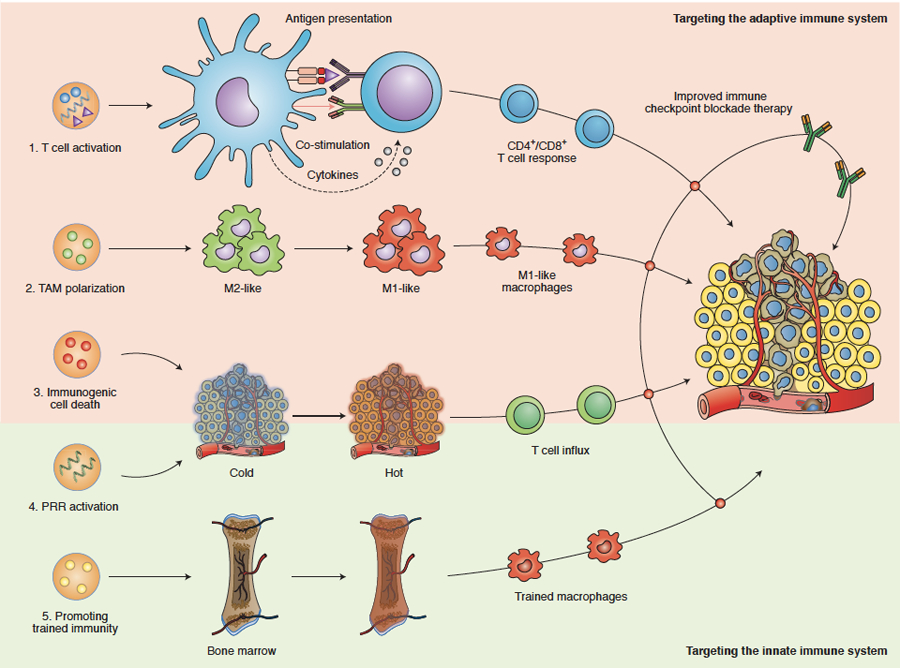
Nanomedicines can be designed to target and modulate components of the adaptive and innate immune systems, thereby improving the outcome of immune checkpoint inhibition therapy. Adaptive immune system-targeted approaches include: (1) Directly targeting antigen-presenting cells by using nanomedicines to deliver (RNA encoding for) tumour antigens and/or drug molecules that modulate co-stimulation and cytokine production. These approaches result in anti-tumour effects mediated via generating and/or activating CD4+ and CD8+ T cells. (2) Nanomedicines can change the polarization of tumour-associated macrophages (TAM) from a pro-tumour and anti-immunotherapy (M2-like) phenotype into a more anti-tumour and pro-immunotherapy (M1-like) phenotype. (3) Nanomedicines containing chemotherapeutic drugs, such as doxorubicin and oxaliplatin, can boost the induction of immunogenic cell death (ICD), which helps reprogram immunogenically ‘cold’ tumours into ‘hot’ tumours. Innate immune system-targeted approaches include: (4) Reprogramming tumours into an immunogenically ‘hot’ phenotype via the activation of pattern recognition receptors (PRRs), eliciting a type I interferon response and inducing anti-tumour T cell immunity. (5) Nanomedicines can be developed to deliver drugs to myeloid cells and their progenitors in the bone marrow, resulting in specific metabolic and epigenetic changes. The ensuing myeloid cells’ hyperresponsiveness towards secondary stimuli has become known as “trained immunity” and may help improve the efficacy of (checkpoint inhibition-based) cancer immunotherapy.
