Figure 5.
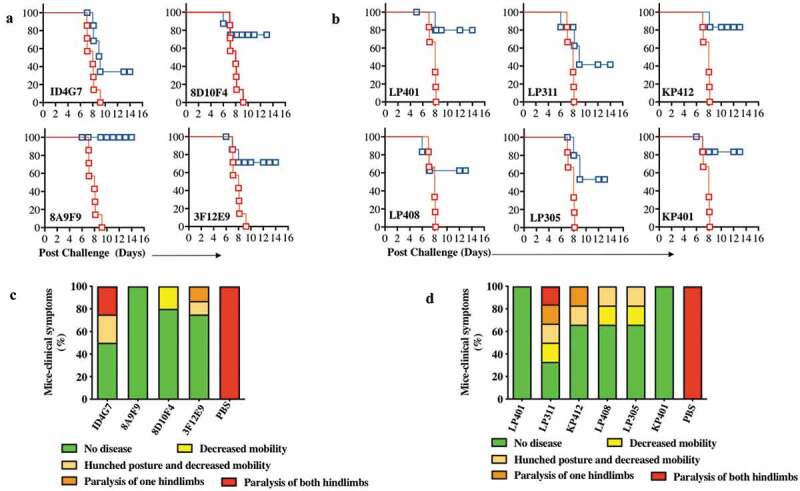
Characterization of ZIKV dMAb protection in vivo in mice from ZIKV.
B6.129S2-Ifnar1tm1Agt/Mmjax (A129) mice (n= 7 per group) were injected with 100 μg of ZIKV dMAb plasmid or an empty pVax1 vector i.m. followed by CELLECTRA® enhanced electroporation delivery 2 d prior to being challenged with 106 plaque-forming units (PFU) of ZIKV PR209. (a and c) ZIKV dMAb clones tested for this challenge, ID4G7, 8D10F4, 8A9F9, and 3F12E9, were derived from ZIKA prME1 DNA-vaccinated murine spleen. (b and d) ZIKV dMAb clones tested for this challenge, LP401, LP408, LP305, LP311, KP401, and KP412 were derived from ZIKV-infected RhMac peripheral blood B cells and spleen. (a) A Kaplan-Meier survival curve of murine ZIKV challenge 2 d prior to mouse-derived ZIKV dMAb administration. Red lines show pVax1-injected cohort, and blue lines indicate dMAb-injected cohort. (b) A Kaplan-Meier survival curve of murine ZIKV challenge 2 d prior to RhMac-derived ZIKV dMAb administration. Red lines show pVax1-injected cohort, and blue lines indicate dMAb-injected cohort. (c-d) Bar graphs of ZIKV-associated clinical scores post challenge at D 9. Each mouse was scored from 1 (green, no disease) to 5 (red, paralysis of both hindlimbs) and recorded as a percentage. The body weight and clinical signs of symptoms were examined and documented daily post challenge. The disease burden was the most severe between D 7 and D 9, where all mice in the control group exhibited a paralysis of both hind limbs or in a moribund state. Non-surviving or moribund mice by D 9 were recorded as 5 (red, paralysis of both hindlimbs) (c) Percent of clinical symptoms in ZIKV-PR209 challenged A129 mice at D 9 receiving mouse-derived anti-ZIKV dMAb clones or pVax1 empty vector control. (d) Percent of clinical symptoms in ZIKV-PR209 challenged A129 mice at D 9 receiving RhMac-derived anti-ZIKV dMAb clones or pVax1 empty vector control.
