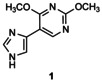
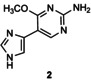
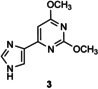
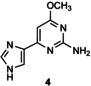
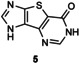
Table 1.
Transglycosylation reactions of flex‐bases (1–5) catalyzed by LlNDT.[a]
|
|
Acceptor |
LlNDT |
Incubation |
Product [%][b] |
||
|---|---|---|---|---|---|---|
|
|
|
[μL] |
time [h] |
Starting |
N1‐glycosylated |
N3‐glycosylated |
|
|
|
|
|
material |
product |
product |
|
1 |
|
1.25 |
3 |
61 |
17 |
22 |
|
2 |
1.25 |
10 |
53 |
28 |
20 |
|
|
3 |
2.5 |
3 |
40 |
74 |
13 |
|
|
4 |
2.5 |
10 |
24 |
65 |
6 |
|
|
5 |
5.0 |
3 |
15 |
80 |
5 |
|
|
6 |
5.0 |
10 |
10 |
88 |
2 |
|
|
7 |
7.5 |
3 |
12 |
86 |
2 |
|
|
8 |
7.5 |
10 |
10 |
89 |
1 |
|
|
9 |
10.0 |
3 |
11 |
87 |
2 |
|
|
10 |
10.0 |
10 |
10 |
89 |
1 |
|
|
11 |
|
1.25 |
3 |
24 |
30 |
46 |
|
12 |
1.25 |
10 |
17 |
45 |
38 |
|
|
13 |
2.5 |
3 |
18 |
42 |
40 |
|
|
14 |
2.5 |
10 |
10 |
78 |
12 |
|
|
15 |
|
1.25 |
3 |
70 |
30 |
– |
|
16 |
1.25 |
10 |
20 |
80 |
– |
|
|
17 |
2.5 |
3 |
7 |
93 |
– |
|
|
18 |
2.5 |
10 |
4 |
96 |
– |
|
|
19 |
|
1.25 |
0.5 |
0 |
100 |
– |
|
20 |
0.63 |
0.5 |
18 |
82 |
– |
|
|
21 |
0.15 |
3 |
40 |
60 |
– |
|
|
22 |
|
1.25 |
3 |
2 |
62 and 32c |
4 |
|
23 |
1.25 |
10 |
2 |
46 and 44c |
6 |
|
|
24 |
0.63 |
3 |
3 |
71 and 23c |
3 |
|
|
25 |
0.63 |
10 |
2 |
58 and 34c |
6 |
|
[a] Reaction conditions: 1 μmol acceptor in 5 % v/v DMSO, 4 μmol thymidine in 10 mm citrate buffer (pH 6.5; 0.1 mL) in the presence of LlNDT at 37 °C. [b] Percentage conversion was determined by reversed‐phase HPLC analysis of an aliquot of the incubation mixture monitored at 254 nm. [c] Product from glycosylation at both imidazole and pyrimidine nitrogens of 5.