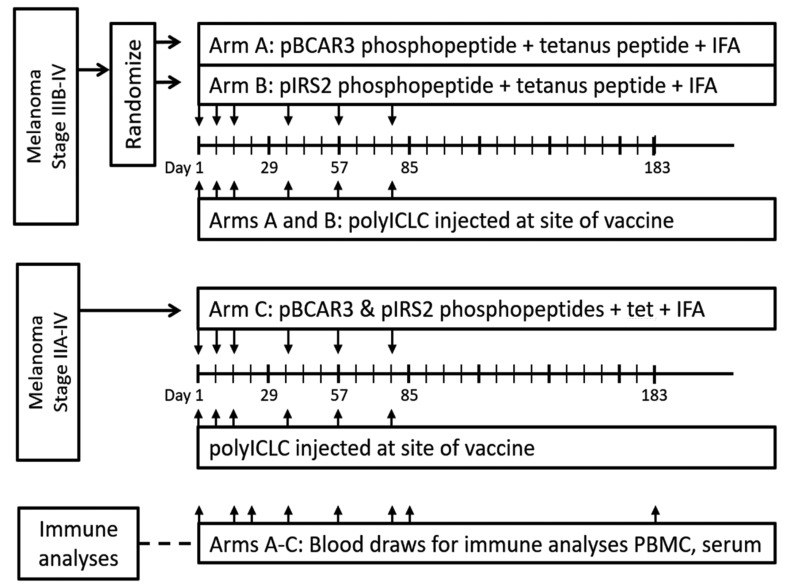
Figure 1.
Design for clinical trial to evaluate safety and immunogenicity of phosphopeptide-based vaccine in melanoma patients. Participants were eligible for all arms with stage IIIB-IV melanoma, with measurable disease, or after surgical resection. Eligibility for arm C was extended to stage II–IIIA patients, after surgical resection. The study was also opened to participants with other solid tumors, but only participants with melanoma enrolled. IFA, incomplete Freund’s adjuvant; PBMC, peripheral blood mononuclear cell; pIRS2, phosphopeptide from insulin receptor substrate 2; tet, tetanus helper peptide.