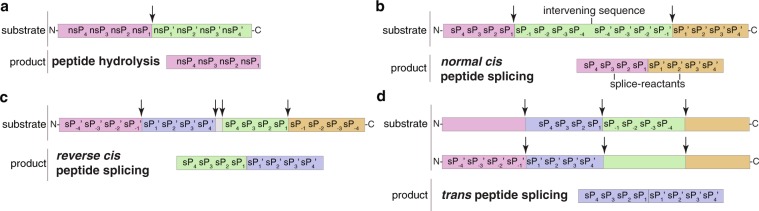
Fig. 1.
Proteasome-catalyzed peptide hydrolysis and peptide splicing. Proteasomes form peptide fragments by: (a) peptide hydrolysis and (b,c) cis peptide splicing, when the two splice-reactants derive from the same polypeptide molecule; peptide fragment ligation can occur in normal order, i.e. following the orientation from N- to C-terminus of the parental protein (normal cis peptide splicing; b), or in reverse order (reverse cis peptide splicing; c); (d) trans peptide splicing, when the two splice-reactants originate from two distinct molecules of the same protein or two distinct proteins. The two fragments, bound together during the peptide splicing reaction, are named splice-reactants. The portion between two splice-reactants is called intervening sequence. In this schematic, we summarize the residue positions (from nsP4 to nsP4’ for non-spliced peptides and from sP4 to sP4’ for spliced peptides) that were examined for the position frequency matrices, and which seem to be relevant in proteasome-catalyzed peptide hydrolysis and peptide splicing reactions. Arrows represent the substrate cleavage sites used by proteasome catalytic Thr1.