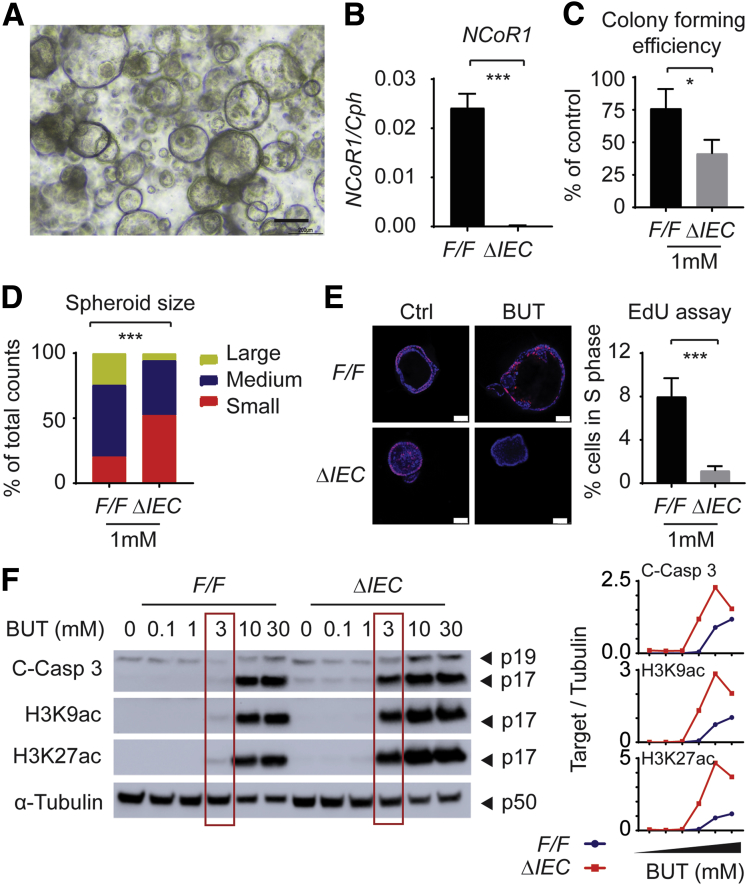
Figure 4.
NCoR1ΔIEC crypts were more sensitive to butyrate-induced suppression of proliferative cells. (A) Image of colonic crypt cells. Scale bar: 200 μm. Images were acquired using a 10× Ph1 ADL (numeric aperture, 0.25) phase-contrast objective on an inverted Nikon Ts2 microscope with a Nikon DS-Fi3 camera and Nikon NIS elements imaging software (Nikon Instruments, Inc, Melville, NY). (B) RT-qPCR of NCoR1 in colonic crypt cells after a few time passages (n = 3). (C) Colony-forming efficiency. Crypt cells were counted and plated at 10 cells/5 μL Matrigel and cultured in media supplemented with 1 mmol/L butyrate. After 5 days, spheroids developed from crypt cells and were enumerated and described as the percentage of control cells with no butyrate treatment. (D) The size of spheroids (diameter) was measured under a microscope, and grouped as large (>200 μm), medium (100–200 μm), and small (<100 μm). The results were described as the percentage of total cell numbers. (E) EdU incorporation assay. Two days after plating, cells were exposed to 1 mmol/L butyrate. Twenty-four hours later, cells were incubated with EdU at final concentration of 10 μmol/L. One hour later, cells were fixed and permeated for immunostaining with EdU Click-iT reaction cocktail containing Alexa Fluor–labeled primary antibody, followed by 4′,6-diamidino-2-phenylindole staining. Images were acquired with a Leica TCS SP5 X confocal microscope and LAS AF imaging software. Scale bar: 50 μm. EdU+ cells were counted and described as the percentage of total cell numbers. (F) NCoR1F/F and NCoR1ΔIEC spheroids were treated with vehicle or 0.1, 1, 3, 10, and 30 mmol/L butyrate for 24 hours. Whole-cell extracts were prepared for electrophoresis and Western blot. Band density of targets and tubulin were quantified using Image Lab 5.2.1 software, and the results are described as the ratio of band density of targets over tubulin. ∗P < .05, ∗∗∗P < .001. BUT, butyrate; C-Casp, cleaved caspase.