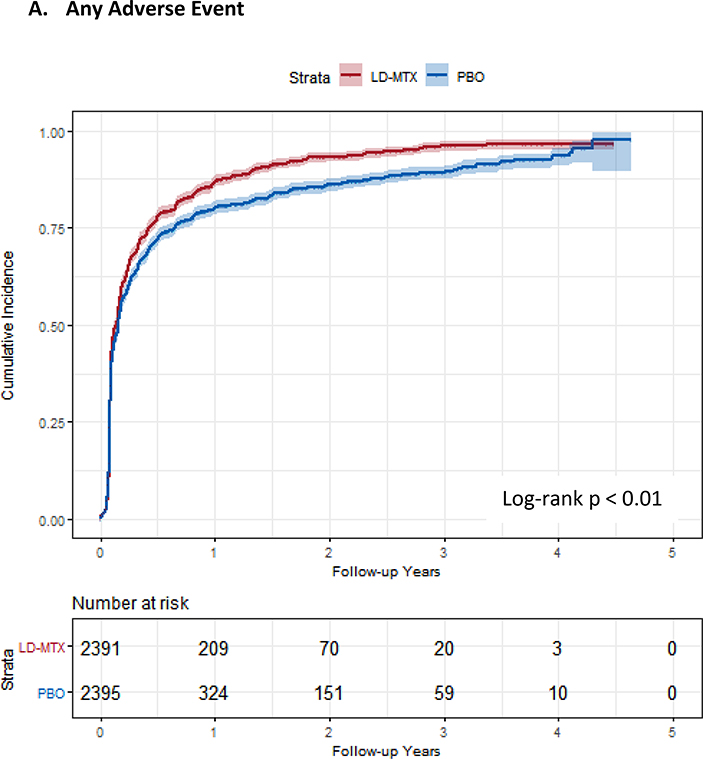
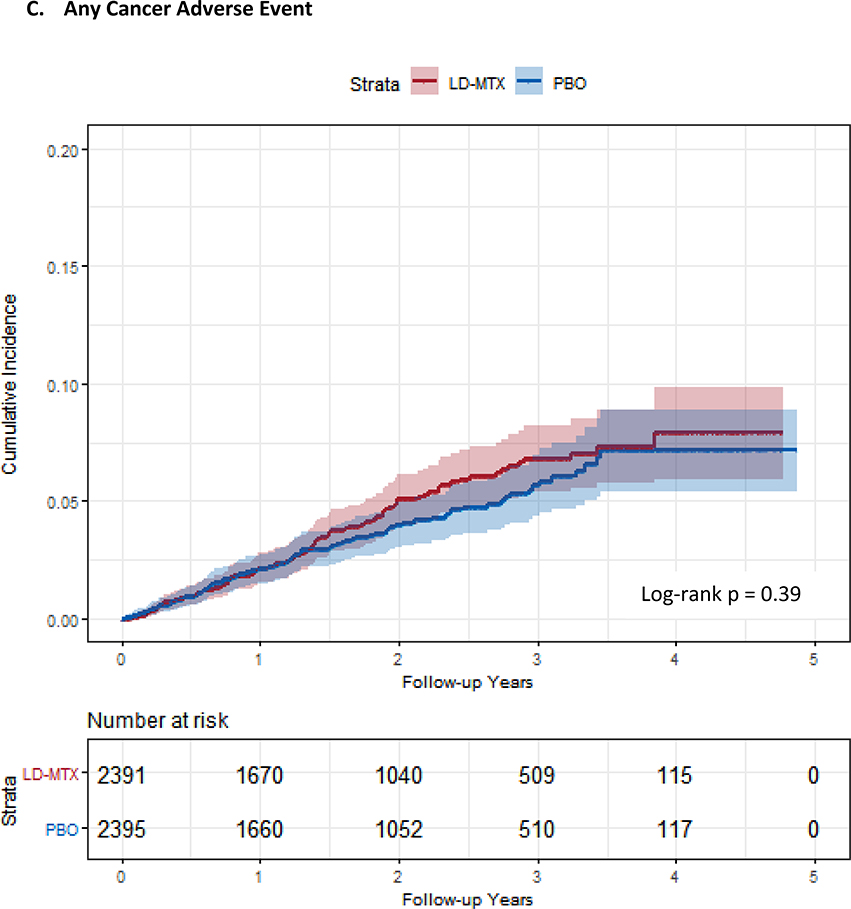
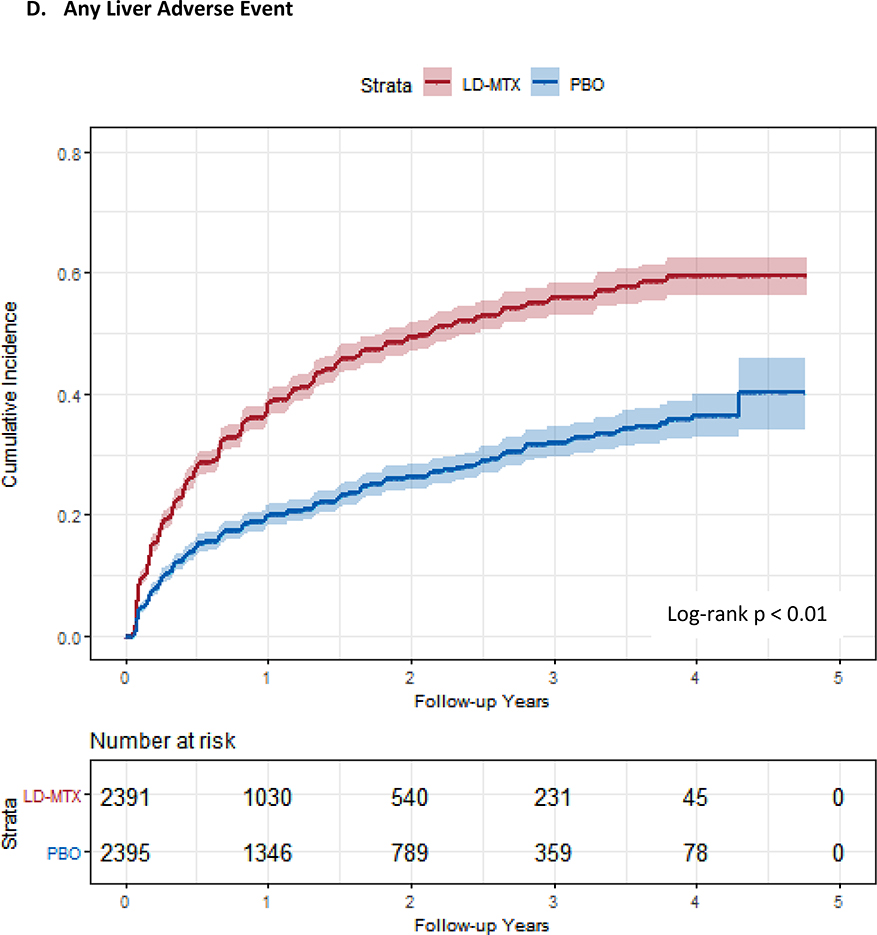
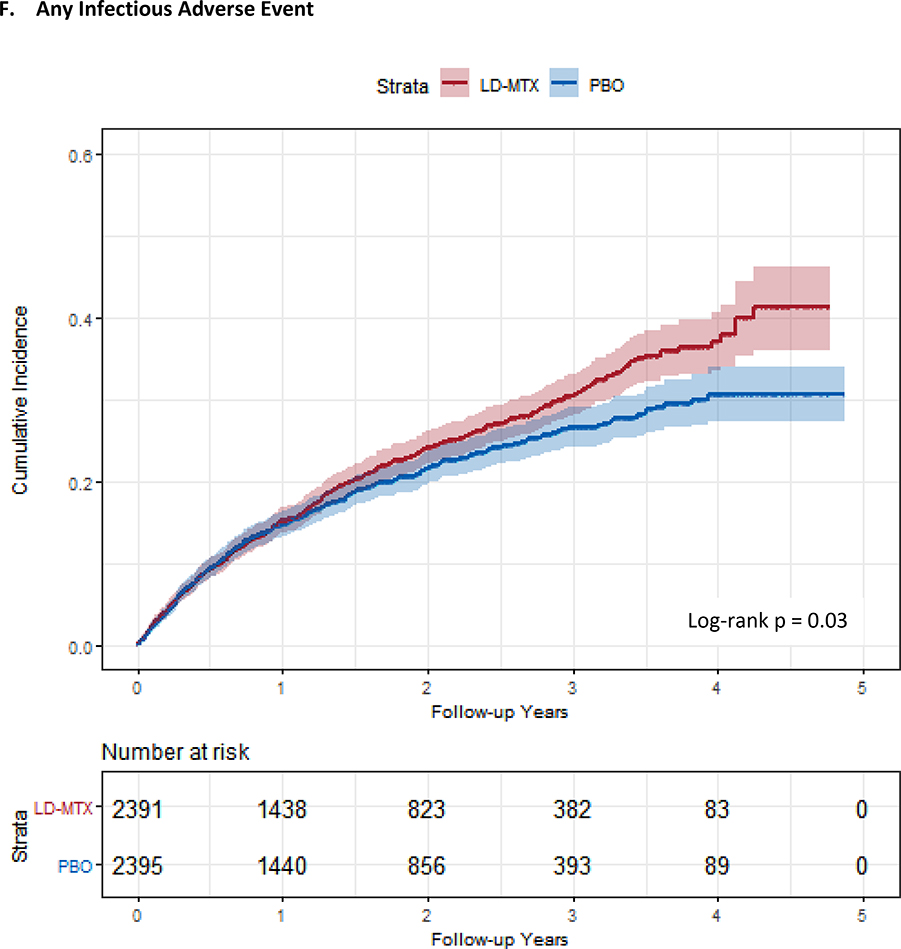
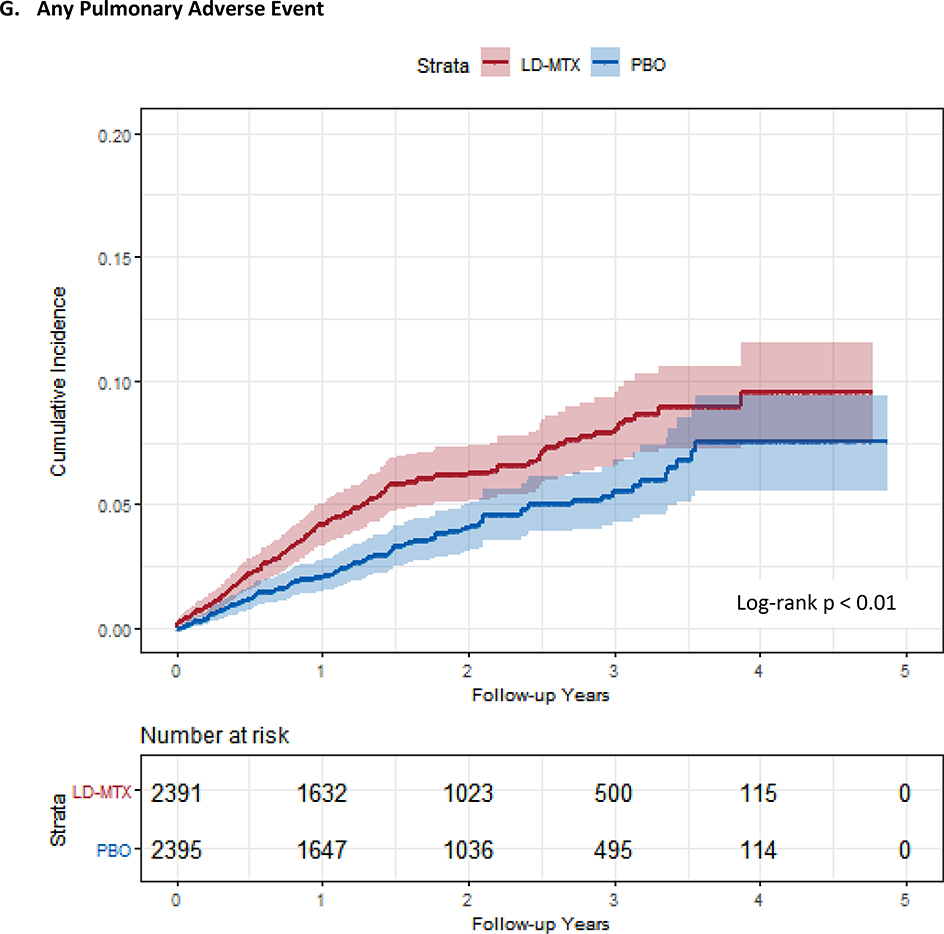
Figure 1:
Cumulative incidence plots with 95% confidence intervals for the modified intention to treat analyses, censoring 180 days after the last dosage of study drug. Log-rank test was used to estimate the p-value. Abbreviations: LD-MTX, low dose methotrexate; PBO, placebo. Panel A: All adverse events of interest with laboratory abnormalities included. Panel B: Any hematologic adverse events with laboratory abnormalities included. Panel C: All cancer adverse events. Panel D: All liver adverse events with laboratory abnormalities included. Panel E: All gastrointestinal adverse events, excluding liver and liver test abnormalities. Panel F: All infectious adverse events. Panel G: All pulmonary adverse events.