Figure 1. Genetic causes of congenital HI.
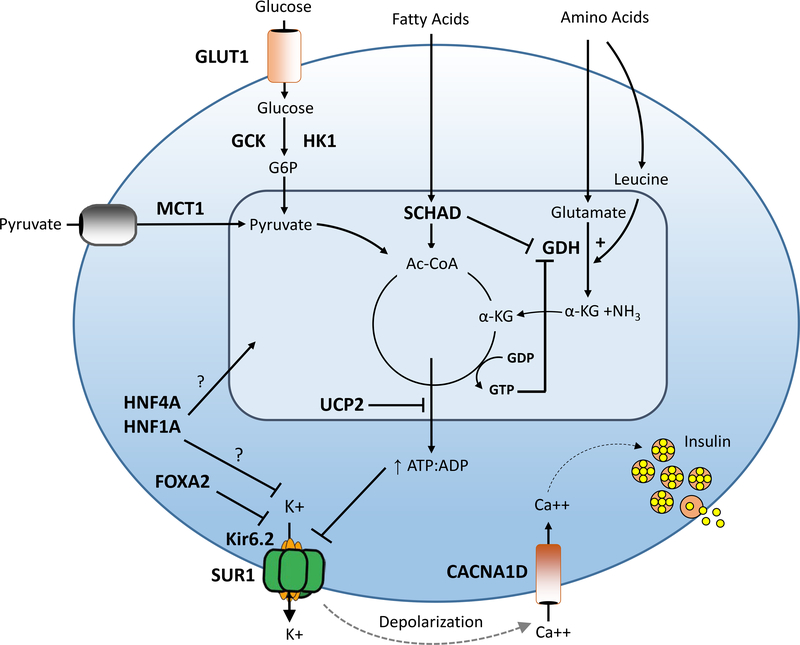
Pancreatic β-cell insulin secretion is predominately controlled by oxidation of glucose and amino acids. Glucose is transported into the β-cell by an insulin-independent glucose transporter (GLUT), predominantly GLUT1, and is phosphorylated by glucokinase (GCK). Glucose metabolism leads to an elevated intracellular ATP/ADP ratio resulting in sequential closure of plasma membrane ATP-sensitive KATP channels (composed of SUR1 and Kir6.2 subunits), membrane depolarization, activation of voltage-gated calcium channels, elevation of cytosolic calcium, and release of insulin from storage granules into the circulation. Amino acids stimulate insulin secretion via a variety of mechanisms. Leucine stimulates insulin secretion by allosterically activating glutamate dehydrogenase (GDH), increasing the oxidation of glutamate to alpha-ketoglutarate which increases the ATP/ADP ratio and triggers the insulin secretion cascade. GDH is allosterically inhibited by GTP and SCHAD. Diazoxide activates KATP channels thereby inhibiting insulin secretion. Defects in the triggering pathway for insulin secretion cause monogenic HI. Genes associated with congenital HI are highlighted in bold and include: SUR1 (sulfonylurea receptor), Kir6.2 (inwardly rectifying potassium channel), GCK (glucokinase), HK1 (hexokinase 1), GDH (glutamate dehydrogenase), SCHAD (short-chain 3-OH acyl-CoA dehydrogenase), HNF4a (hepatocyte nuclear transcription factor 4alpha), and HNF1a (hepatocyte nuclear transcription factor 1alpha), MCT1 (monocarboxylate transporter 1), UCP2 (uncoupling protein 2).