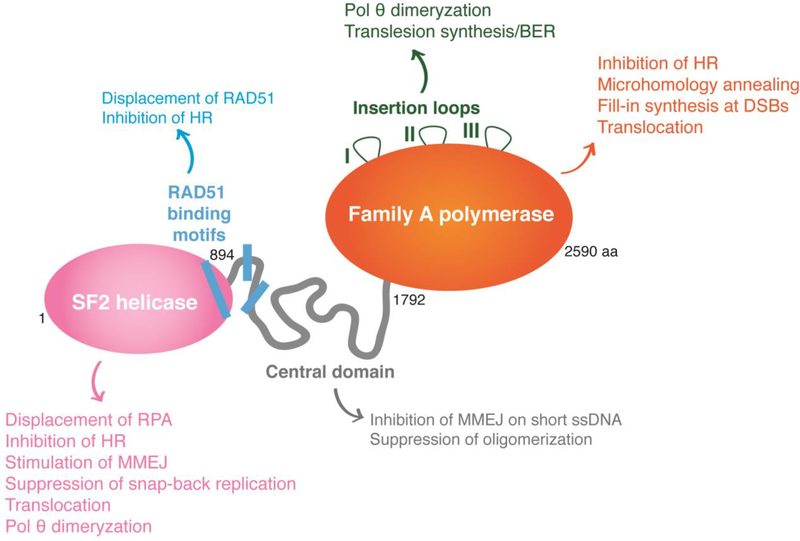
Figure 3. Polθ is a unique multidomain enzyme.
Schematic representation of the different domains of human Polθ, depicting the structure and function of its helicase domain (pink), the unstructured central domain (grey) and the family-A polymerase domain (orange). The helicase domain drives MMEJ activity during chromosomal translocations, promotes Polθ dimerization, and suppresses HR and snap-back replication. The central domain contains three predicted RAD51 binding motifs that are not conserved in mouse. These motifs are implicated in suppression of HR through the inhibition of RAD51 nucleofilament formation. The primary and unequivocal function of the polymerase domain is to perform fill-in synthesis during MMEJ. The polymerase domain has also been reported to participate in tethering DNA ends. The polymerase domain comprised three insertion loops that are essential for Polθ to bypass bulky lesions and abasic sites, and were proposed to promote dimerization.