Abstract
Purpose
Fasting blood homocysteine is increased in PCOS women and is involved in several of its co-morbidities including cardiovascular disease and infertility. Corrective interventions based on the administration of supra-physiologic doses of folic acid work to a low extent. We aimed to test an alternative approach.
Methods
This was a prospective, randomized, parallel group, open label, controlled versus no treatment clinical study. PCOS women aged > 18, free from systemic diseases and from pharmacological treatments were randomized with a 2:1 ratio for treatment with activated micronutrients in support to the carbon cycle (Impryl, Parthenogen, Switzerland—n = 22) or no treatment (n = 10) and followed-up for 3 months. Fasting blood homocysteine, AMH, testosterone, SHBGs, and the resulting FTI were tested before and at the end of the follow-up.
Results
The mean baseline fasting blood homocysteine was above the normal limit of 12 μMol/L and inversely correlated with SHBG. AMH was also increased, whereas testosterone, SHBG, and FTI were within the normal limit. The treatment achieved a significant reduction of homocysteine, that did not change in the control group, independently of the starting value. The treatment also caused an increase of AMH and a decrease of SHBGs only in the subgroup with a normal homocysteine at baseline.
Conclusions
In PCOS ladies, blood homocysteine is increased and inversely correlated with the SHBGs. Physiologic amounts of activated micronutrients in support to the carbon cycle achieve a reduction virtually in all exposed patients. Whether this is of clinical benefit remains to be established.
Keywords: Homocysteine, Micronutrients, One carbon cycle, PCOS
Introduction
Polycystic ovary syndrome (PCOS) is a heterogeneous endocrine disorder characterized by irregular menses, hyperandrogenism, and polycystic ovaries affecting women of reproductive age. Its prevalence ranges from 6 to 20% according to the diagnostic criteria used [1]; however, it is the most common female endocrine disorder. Although the clinical manifestations of PCOS are variable, its co-morbidity with androgen excess, insulin resistance, obesity, and metabolic syndrome clearly points to a main metabolic component of the disease. It has been well shown that circulating markers of oxidative stress are abnormal in women with PCOS with an average increase of 23% of blood fasting homocysteine (Hcy) [2].
A possible reason for Hcy increase in PCOS is the higher insulin level resulting from insulin resistance. Insulin down regulates the transcription of cystathionine beta synthase (CBS), the main enzyme for Hcy elimination [3]. However, increased Hcy in PCOS is not related to degree of insulin resistance (IR), as well as to obesity or androgen levels [4]. Moreover, lowering the insulin concentration in PCOS patients with the drug metformin does not result also in lower Hcy, rather a significant increase has been reported [5, 6], which points to the contribution of other genetic and/or dietary and/or metabolic factors besides the possible role of insulin.
Hyperhomocysteinemia (HHcy) has been linked to a variety of diseases including cardiovascular disease, neurodegeneration, diabetes, and cancer [7]. Not surprising, HHcy has been recognized since long time also as a main marker of both female and male reproductive dysfunction [8], which generates further interest in the role of Hcy in reproductive issues of PCOS patients. A higher amount of Hcy in follicular fluid of PCOS women undergoing oocyte pick-up following FSH stimulation sharply marked lower oocyte quality, fertilization rates, embryo quality, and pregnancy rates with no pregnancies occurring from oocytes whose follicles contained more than 8 μmolar Hcy [9]. Interestingly, the blood Hcy of the same patients was normal (11.7 ± 2.9 μMol) suggesting the occurrence of a follicle-specific impairment of Hcy metabolism in PCOS and a main role of Hcy in PCOS subfertility also in women with normal Hcy blood values. High blood Hcy was also found to be the strongest predictor of recurrent pregnancy loss in PCOS women [10]; thus, the negative effect of HHcy may also affect the post-conceptional development.
PCOS may also be associated with increased incidence of cardiovascular disease and this risk should be considered even in young women with PCOS [11]. Surrogate markers of cardiovascular disease are already altered in adolescent PCOS ladies and metformin treatment positively modified these markers leading to the proposal of a preventive treatment [12]. However, the impairment of Hcy metabolism resulting from metformin treatment [5, 6] may lead to the opposite long-term outcomes. Indeed, it has been calculated that each increase of 5 µmol/L in Hcy level increases the risk of CHD events by approximately 20%, independently of traditional CHD risk factors [13].
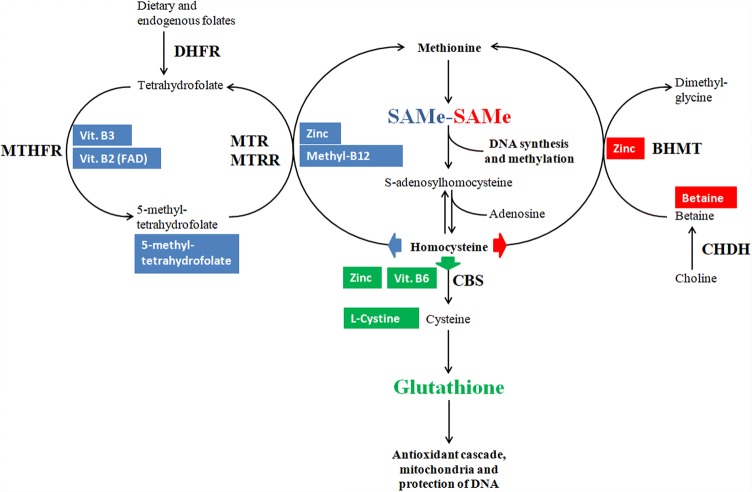
Excess of circulating Hcy is removed by three alternative pathways: Hcy re-methylation to methionine by a methyl group donated by either methyl-tetrahydrofolate or betaine and Hcy transsulfuration to cysteine, which can be then used for the synthesis of the universal cellular antioxidant glutathione [14]. Imbalances of any of the above pathways result in accumulation of Hcy and it is very difficult to understand which of the pathways is responsible for HHcy in the single case. Accordingly, a combined supplementation supporting both the re-methylation (folates, B2, B3, B12, betaine, and zinc) and the transsulfuration (B6, cysteines, and zinc) of Hcy may improve the disposal of Hcy by all available pathways independently of the specific and unknown imbalance occurring in the single subject (see Fig. 1 for details). In addition, the availability of folates and B12 in their activated forms, i.e., methylfolate and methylcobalamin, may increase their actual metabolic suitability by escaping the common genetic variants of the respective activating enzymes Methylene TetraHydroFolate Reductase (MTHFR) and Methionine Synthase Reductase (MTRR) [15, 16].
Fig. 1.
Rationale for homocysteine removal by the administration of a full array of activated micronutrients. The one carbon cycle is regulated by the availability of its substrates; accordingly, a wider availability of each substrate may enhance its performance. Blue background substrates: Folates, B12, chelated zinc, Vit. B2 and Vit. B3 feed homocysteine re-methylation by the folate pathway; Folates in the form of methylfolate escape possible genetic defects in the activity of DHFR and MTHFR; B12 in the form of methylcobalamin escapes possible genetic defects in the activity of MTRR. Red background substrates: Betaine and chelated zinc feed homocysteine re-methylation by the betaine pathway; Pre-formed betaine escapes possible genetic defects in the activity of CHDH and provide an excess of substrate for the BHMT reaction. Green background substrates: Vit. B6, chelated zinc and cysteines feed homocysteine transsulfuration to glutathione (GSH); Excess of cysteines downstream to CBS reaction ensure GSH production even in case of genetic defects of CBS. Key enzymes subject to genetic variability: DHFR DiHydroFolate reductase, MTHFR methylenetetrahydrofolate reductase, MTR methionine reductase (methionine synthase), MTRR methionine synthase reductase, CBS cystathionine beta synthase
The present study was intended to explore the effect of an Hcy-targeted supplement containing a full range of activated micronutrients in support to the carbon cycle (Impryl, Switzerland) in women with PCOS independently of their fasting blood Hcy baseline levels.
Methods
This was a prospective, randomized, parallel group, open label, controlled versus no treatment clinical study. The study was approved by the Ethical Committee of Aziende Sanitarie della Regione Umbria (CEAS).
We enrolled women referring for gynaecological problems and diagnosed as affected by PCOS according to Rotterdam criteria [17], aged more than 18 and delivering a written informed consent to the study. Criteria for exclusion were ongoing pregnancy, ongoing pharmacological treatment (oral antidiabetic drugs, insulin, antihypertensives, and any hormone), and ongoing systemic or endocrine diseases including hypertension and thyroid diseases.
The enrolled patients were randomized according to a computer-generated randomization list for treatment with the test product or no treatment with a 2:1 ratio. Patients assigned to the active intervention group assumed one tablet per day of an oral supplement containing essential or semi-essential micronutrients in support to the one carbon cycle: betaine 200 mg, l-cystine 200 mg, chelated zinc 10 mg, niacin (vit. B3) 16 mg, pyridoxine (vit. B6) 1.4 mg, riboflavin (vit. B2) 1.4 mg, 5-methyl-tetrahydrofolate 400 μg, and methylcobalamin (vit. B12) 2.5 μg (Impryl, Parthenogen, Switzerland).
All patients were tested for fasting blood Hcy, Anti-Mullerian Hormone (AMH), circulating testosterone, and steroid hormone-binding globulins (SHBG) at baseline and after a 3-month follow-up period. The analyses were performed at the hospital reference lab according to routine methodologies and the Free Testosterone Index (FTI) was calculated based on blood testosterone and SHBG values.
The differences in quantitative variables at start of study between treatment groups were assessed by the Student’s t test. Pearson correlation coefficient was used to determine the relationship between variables and paired categorical data were analyzed by McNemar test. Comparisons within and between groups over time were analyzed by mixed model repeated measures analysis of variance (RM ANOVA). “Time” (variable values at basal and 1st follow-up) and “group” (treatment arms) were considered as within-subjects and between-subjects factors, with two “time” and two “group” levels, respectively. To interpret the interaction term effect, simple main effects of time within each treatment condition were also examined. All statistical analyses were performed using IBM-SPSS® version 25.0 (IBM Corp., Armonk, NY, USA, 2017). In all analyses, a two-sided p value < 0.05 was considered significant.
Results
A total of 33 PCOS ladies (mean age 26.8, range 19–35) were enrolled between June 2017 and October 2018, and one patient withdrew her participation for personal reasons after the basal visit. Out of 32 patients randomized, 22 entered the active treatment group, and 10 the no treatment group. The average treatment/follow-up duration was 93 days (range 83–105 days). Treatment compliance was good or very good in the majority of treated patients with no occurrence of adverse events.
The baseline values for the tested variables are reported in Table 1. The baseline mean values of testosterone, SHBG, and FTI were within the upper normal limit with only 5 patients (15.6%) exerting an elevated FTI. The mean values of basal AMH (11.09 ng/ml) were above the normal limit with 16 out of 30 patients (53%) exerting an abnormal value. Mean basal AMH increased with age (Pearson r = 0.46, p = 0.028). Mean fasting blood Hcy (12.12 μMol/L) was slightly above the upper normal limit with 13 patients (40%) reporting abnormal values and inversely correlated with SHBG (Pearson r = − 0.358, p = 0.048). There was no correlation between Hcy and the other tested variables (data not shown).
Table 1.
Baseline laboratory values, all patients
| Variable | Unit | n | Mean values ± SD (range) | Normal range | Pts out of normal range | |
|---|---|---|---|---|---|---|
| AMH | ng/ml | 31 | 11.09 ± 6.4 (2.45–23.0) | 0.9–9.5 | 16 | 52.0% |
| Testosterone | ng/dl | 33 | 54.76 ± 20.7 (21–100) | 8–60 | 14 | 42.0% |
| SHBG | nmol/L | 32 | 55.69 ± 24.4 (21.2–106) | 15–103 | 1 | 3.1% |
| FTI | units | 32 | 4.07 ± 2.3 (1.01–9.03) | < 7 | 5 | 15.6% |
| Homocysteine | μMol/L | 33 | 12.12 ± 4.9 (4.2–24.3) | 6–12 | 13 | 39.4% |
Weight and height data for the calculation of the body mass index (BMI) were available only for 22 of the enrolled patients. Their mean BMI was within the normal limit (22.8, range 19.0–38.6). Only 6 of them had a BMI above the limit of 25. BMI did not correlate with AMH (Pearson r = − 0.12, p = 0.58), whereas it showed negative correlation with SHBG (Pearson r = − 0.56, p = 0.007). Compared to those with BMI within the normal limit, patients with BMI > 25 exerted significantly lower baseline SHBG resulting in a significant increase of the calculated FTI. In addition, they had a strong trend for a lower AMH value at enrolment (p = 0.054), see Table 2. BMI did not mark any difference for testosterone and Hcy.
Table 2.
Baseline laboratory values according to BMI
| Group | n | AMH | Testosterone | SHBG | FTI | Homocysteine |
|---|---|---|---|---|---|---|
| ng/ml | ng/dl | nmol/L | Units | μMol/L | ||
| BMI < 25 | 16 | 11.45 ± 5.0 | 50.83 ± 10.3 | 61.89 ± 21.1 | 3.09 ± 1.7 | 12.99 ± 6.0 |
| BMI > 25 | 6 | 6.55 ± 4.9 | 49.44 ± 19.0 | 30.0 ± 9.9 | 6.34 ±2.1 | 10.35 ± 3.3 |
| p | 0.054 | 0.87 | 0.002 | 0.001 | 0.32 | |
Significant p values are shown in bold
After 3 months of follow-up, the mean values of AMH were significantly increased (p = 0.029) in the treatment group and significantly decreased (p = 0.021) in the control group. The difference between subjects over time was also significant (p = 0.002). Circulating testosterone did not change in the treatment group, but significantly decreased (p = 0.015) in controls with a significant difference over time within subjects (p = 0.018) and between subjects (p = 0.012); however, SHBG and FTI remained unchanged in both groups.
The treatment with the test micronutrients significantly reduced the mean fasting blood Hcy from 11.91 to 8.12 μMol/L (p = 0.003), whereas Hcy did not change in the control group (from 11.38 to 12.11 μMol/L, n.s.) with a significant difference between subjects over time (p < 0.017). The reduction of Hcy was likely of clinical significance due to its amplitude (> 30% average reduction) and to the fact that 7 out of 8 treated patients having a high Hcy at baseline recorded a value within the normal limit after treatment. Worth to note, the treatment with micronutrients resulted in a lower Hcy value in all treated patients independently of their baseline value. The only patient whose Hcy was not reduced after treatment (from 6.3 to 7.2) stated a good treatment compliance; however, her values were already at the lower limit of normal range and were difficult to reduce. The outcomes at the end of follow-up for both the treatment and the control group are depicted in Table 3.
Table 3.
Treatment outcomes
| Variable | Unit | Treated | Controls | RM ANOVA | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Pre | Post | Change (%) | Simple main effect p | N | Pre | Post | Change (%) | Simple main effect p | Within subjects factor p | Between-subjects factor p | ||
| AMH | ng/ml | 20 | 10.74 ± 6.6 | 12.17 ± 7.1 | 13.30 | 0.029 | 10 | 11.8 ± 6.4 | 9.95 ± 6.4 | − 15.7 | 0.021 | 0.674 | 0.002 |
| Testost. | ng/dl | 22 | 53.91 ± 22.9 | 54.34 ± 22.9 | 0.8 | 0.878 | 10 | 55.2 ± 16.7 | 41.5 ± 19.0 | − 24.8 | 0.015 | 0.018 | 0.012 |
| SHBG | nM/L | 21 | 58.79 ± 23.8 | 55.5 ± 23.8 | − 5.6 | 0.27 | 10 | 52.1 ± 25.7 | 50.58 ± 28.1 | − 2.90 | 0.662 | 0.326 | 0.716 |
| FTI | units | 22 | 3.94 ± 2.4 | 3.98 ± 2.9 | 5.80 | 0.942 | 10 | 4.33 ± 1.9 | 3.51 ± 2.1 | − 19.9 | 0.196 | 0.363 | 0.320 |
| Hcy | μM/L | 21 | 11.91 ± 4.9 | 8.12 ± 2.3 | − 31.8 | 0.003 | 10 | 11.38 ± 4.9 | 12.11 ± 6.4 | 6.40 | 0.498 | 0.096 | 0.017 |
| Hcy > 12 | 21 | 8 | 1 | − 33.3 | 0.023 | 10 | 3 | 3 | 0 | 1.000 | |||
| 30.00% | 30.00% | ||||||||||||
| 38.10% | 4.80% | ||||||||||||
Significant p values are shown in bold
The treatment was effective in lowering Hcy independently of its baseline value (Table 4). Patients with a baseline Hcy below the upper normal limit exerted a 15% reduction of their value (p = 0.04) compared to a 46% reduction (p = 0.01) in those with an elevated starting value. The difference over time was significant both within subjects (p < 0.0001) and between subjects (p = 0.003). Patients with Hcy within the normal limit at enrolment exerted a significant reduction of circulating SHBG after treatment (− 11.3%, p = 0.04) compared to a non-significant increase (5.9%) in those starting with an elevated Hcy level.
Table 4.
Treatment outcomes according to baseline homocysteine values
| Variable | Unit | Basal homocysteine < 12 μMol/L | Basal homocysteine > 12 μMol/L | RM ANOVA | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Pre | Post | Change (%) | Simple main effect p | N | Pre | Post | Change (%) | Simple main effect p | Within subjects factor p | Between subjects factor p | ||
| AMH | ng/ml | 13 | 11.05 ± 7.6 | 12.21 ± 8.1 | 10.50 | 0.171 | 8 | 10.17 ± 4.6 | 12.1 ± 5.5 | 19.00 | 0.090 | 0.028 | 0.555 |
| Testost. | ng/dl | 13 | 50.95 ± 17.4 | 54.34 ± 22.9 | 6.70 | 0.713 | 8 | 54.25 ± 29.2 | 55.63 ± 28.0 | 2.50 | 0.753 | 0.949 | 0.644 |
| SHBG | nM/L | 13 | 63.43 ± 25.4 | 56.26 ± 21.4 | − 11.3 | 0.020 | 8 | 51.24 ± 20.2 | 54.25 ± 28.8 | 5.90 | 0.622 | 0.472 | 0.088 |
| FTI | units | 13 | 3.49 ± 2.2 | 3.96 ± 2.7 | 13.50 | 0.139 | 8 | 4.04 ± 2.3 | 4.51 ± 3.2 | 11.60 | 0.630 | 0.91 | 0.317 |
| Hcy | μM/L | 13 | 8.98 ± 1.4 | 7.6 ± 1.8 | − 15.4 | 0.036 | 8 | 16.6 ± 4.8 | 8.98 ± 2.9 | − 45.9 | 0.010 | < 0.0001 | 0.003 |
Significant p values are shown in bold
Discussion
The average blood fasting Hcy of our patients was slightly above the normal limit with individual values ranging from low (4.2 μMol/L) to clinically significant HHcy (24.3 μMol/L), which may well represent the situation in an unselected PCOS population [18]. The level of Hcy was inversely related to the SHBGs, but did not correlate with the other study variables.
The treatment with activated micronutrients in support to the one carbon cycle (Impryl, Parthenogen, Switzerland) was very effective in lowering blood fasting Hcy in PCOS ladies. The effect was significant independently of the starting level with some Hcy reduction in all but 1 of the treated patients (a patient with very low starting concentration), i.e., there were no resistant subjects in the cohort of concern. This wide responsiveness is likely due to the availability of support to all pathways for Hcy elimination and to the neutralization of the most common defective genetic variants of the concerned enzymes by means of activated micronutrients downstream to the possible genetic blockades. Worth to note, there were no patients ending with an Hcy value below the normal limits, i.e., the treatment effect appears self-limited within the physiologic boundaries.
These outcomes positively compare to previous attempts of HHcy treatment in PCOS women, where plain folic acid supplements were tested. Asemi et al. assessed obese PCOS ladies and found that only 5 mg per day of folic acid, i.e., 25 folds the established daily need of 200 μg, was effective in lowering blood Hcy compared to placebo, while the smaller dose of 1 mg daily, i.e., fivefolds the daily need, did not [19]. This need for huge supra-physiologic doses indicates that folic acid alone is not a specific treatment. It may also carry safety risks. Folic acid is a synthetic derivate easy and cheap to manufacture, but does not well feed the natural human pathway. It must be activated with a double reductive passage by the enzyme DiHydroFolate Reductase (DHFR) to which it has a very low affinity, so that at concentrations above 2 μMol, it behaves as a competitive inhibitor [20]. This may lead to the “pseudo MTHFR syndrome”, where patients treated with high doses of folic acid experience a paradox blockade of the pathway [21]. Any folic acid not activated by DHFR may enter circulation as UnModified Folic Acid (UMFA), which has been linked to a variety of possible negative outcomes including derangement of immunity, neurodegeneration, and cancer, although a direct causal link cannot be stated [22]. Our treatment included instead a standard dose of the physiologic, natural form of activated folate, i.e., 5-methyl-tetrahydrofolate. This does not need activation by DHFR and MTHFR and has been shown to be better effective than folic acid in the repletion of folate stores independently of the genetic background [23]. In addition, plain folic acid treatment supports only one of the three pathways for Hcy detoxification and may not work properly anytime the other pathways are concerned. A previous clinical trial aimed at correcting the HHcy induced by metformin in PCOS patients found that metformin did indeed significantly increase circulating Hcy and that folic acid reduced it only by 8% compared to a 21% reduction with a multivitaminic approach (B1, B6, and B12) [24]. The wide and strong response of blood Hcy to our treatment further endorse the concept that Hcy lowering in PCOS, if indicated, should be attempted with a multinutrient approach aimed at supporting all the pathways of concern.
Due to the small sample size, our explorative study does not allow detailed comments on the effect of treatment on the global metabolic control of PCOS. Moreover, we enrolled young (19–35 yo) and lean (average BMI = 22.8) PCOS ladies with a mild form of disease exerting no androgenic activation and only a modest elevation of the AMH level, i.e., with little defects suitable for improvement.
At the end of the 3-month follow-up period, the control group recorded a significant reduction of testosterone, which remains an unexplained finding. However, androgen activation in PCOS should be assessed by a full androgen hormone profile plus grading of hirsutism [25] and isolated fluctuations of testosterone may not reflect true changes.
A combination of micronutrients similar to the one here tested administered to infertile women with low ovarian reserve achieved a significant increase of their AMH level and resulted in improved fertility with spontaneous pregnancies occurring in ladies waiting for oocyte donation [26]. Aiming to confirm this effect also in PCOS ladies, we included AMH among the tested parameters and found that also in our study, the treatment group recorded an increase of the AMH level. Women with PCOS exert an increased release of AMH and this is considered as part of the pathogenic mechanisms of the disease [27]. Based on this view, the increase of AMH should be assumed as a negative treatment outcome. However, increased AMH in PCOS may depend on both an increased number of secreting follicles, likely creating problems, and an increased release from single follicles, which may mark a better performance of some follicles. Thus, it is still possible that the increase of AMH induced by micronutrients may improve the fertility also in PCOS ladies, which should be tested in an infertile PCOS population.
Circulating SHBGs are consistently decreased in PCOS contributing to the increased FTI [28] and the decrease correlates with insulin resistance [29]. The baseline Hcy values of our patients correlated negatively with the concentration of SHBG, which is a new finding of this study. The treatment resulted in a significant decrease of SHBG in the subgroup of patients without increased Hcy at baseline, whereas it increased non-significantly in those with abnormal Hcy. This is a strong argument in favour of a direct link between Hcy and SHBG derangement in PCOS.
Assumed that a multiple micronutrient support is effective in reducing Hcy, whether or not a Hcy lowering treatment should be offered to PCOS ladies remains a controversial question. The function of the one carbon metabolism is deranged in PCOS possibly resulting in impaired mitochondrial activity [30] and the resulting abnormal DNA methylation and epigenetics may contribute to the pathogenesis of the disease [31], which could be a strong reason to correct HHcy in any PCOS patient. Moreover, Hcy is a well-known risk factor for cardiovascular disease [13, 32] that is a main co-morbidity of PCOS [33], hence a preventive treatment may be indicated. However, large size, prospective randomized trials failed to prove any benefit from folic acid supplementation in cardiovascular disease [34, 35] and the same could apply also to PCOS.
Due to its positive effect on insulin resistance, metformin is often prescribed to PCOS ladies and its use for the prevention of the cardiovascular risk in young PCOS ladies has also been proposed [12]. However, metformin treatment per se is known to increase blood Hcy [36], and in the long term, it may result in negative outcomes. The increase of Hcy under metformin is likely linked to perturbances to the metabolism of vitamin B12 and may be more evident in obese PCOS [6]. Co-treatment with folic acid has been shown to be effective in preventing the Hcy increase and was associated with a better metabolic control [5], i.e., besides the safety gain the co-treatment may also result in better metformin efficacy. Accordingly, a one carbon cycle support should always be considered in PCOS ladies treated with metformin and the combination of micronutrients of this study qualifies as a suitable tool.
Finally, a Hcy lowering treatment should be considered in PCOS ladies suffering from reproductive problems. Hcy is a well-known enemy of reproductive functions [8] and its role in PCOS infertility seems to be very strong. Independently of the blood levels, and the Hcy concentration in the follicular fluid of infertile PCOS women is higher than controls [18]. A study investigating the Hcy concentration in the mono-follicular fluid of PCOS women undergoing Assisted Reproduction Technologies (ART) showed strong inverse correlation of follicular Hcy with the oocyte quality, embryo quality, and pregnancy rates [9]. The correlation was so strong that no pregnancies occurred in women, whose follicular Hcy exceeded 8 μMol/L. In the same patients, the blood Hcy concentration did not correlate with the outcomes, meaning that a patient with a normal blood Hcy may still suffer from an excess of follicular Hcy. Within this perspective, a reduction of follicular Hcy might benefit every infertile PCOS patient; however, the ability of the micronutrients to benefit also follicular Hcy should be tested in duly designed studies.
In summary, the present study confirms that an unselected population of PCOS women exert an average Hcy above the normal limit, with values ranging from low to pathologic and that a nutritional supplementation with physiologic doses of activated micronutrients in support to the one carbon cycle decreases their Hcy independently of the starting values and normalizes it virtually in all patients. Whether this treatment is of benefit to the metabolic control of the disease and to the reproductive potential of these women remains to be investigated by duly designed enlarged clinical trials.
Author contributions
TB, MD, AF, and SG contributed to the study conception and design. Material preparation, data collection, and analysis were performed by NS, AC, MCA, and MR, and statistical analysis was done by VB. The first draft of the manuscript was written by MD and NS and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
This study was supported by a grant of University of Perugia (Fondo di finanziamento della ricerca di base del Dipartimento di Scienze Chirurgiche e Biomediche, year 2015) and registered in ISRCTN number 14115156.
Compliance with ethical standards
Conflict of interest
M. D. reports personal fees from Parthenogen SAGL, outside the submitted work; in addition, M. D. is inventor of the pending patent ‘Dietary supplementation to achieve oxy-redox homeostasis and epigenetic stability’. The other authors have no competing interests to disclose.
Ethical approval
The research was carried out according to the principles of the Declaration of Helsinki, in accordance with the relevant guidelines and ethical regulations in research involving human participants. The study was approved by the Ethical Committee of Aziende Sanitarie della Regione Umbria (CEAS).
Informed consent
Each enrolled subject delivered a written informed consent for participation in the study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sirmans SM, Pate KA. Epidemiology, diagnosis, and management of polycystic ovary syndrome. Clin Epidemiol. 2014;6:1–13. doi: 10.2147/CLEP.S37559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murri M, Luque-Ramırez M, Insenser M, Ojeda-Ojeda M, Escobar-Morreale H. Circulating markers of oxidative stress and polycystic ovary syndrome (PCOS): a systematic review and meta-analysis. Hum Reprod Update. 2013;19(3):268–288. doi: 10.1093/humupd/dms059. [DOI] [PubMed] [Google Scholar]
- 3.Ratnam S, Maclean KN, Jacobs RL, Brosnan ME, Kraus JP, Brosnan JT. Hormonal regulation of cystathionine beta-synthase expression in liver. J Biol Chem. 2002;277(45):42912–42918. doi: 10.1074/jbc.M206588200. [DOI] [PubMed] [Google Scholar]
- 4.Meng Y, Chen X, Peng Z, Liu X, Sun Y, Dai S. Association between high serum homocysteine levels and biochemical characteristics in women with polycystic ovarian syndrome: a systematic review and meta-analysis. PLoS One. 2016;11(6):e0157389. doi: 10.1371/journal.pone.0157389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Palomba S, Falbo A, Giallauria F, Russo T, Tolino A, Zullo F, Colao A, Iorio F. Effects of metformin with or without supplementation with folate on homocysteine levels and vascular endothelium of women with polycystic ovary syndrome. Diabetes Care. 2010;33(2):246–251. doi: 10.2337/dc09-1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Esmaeilzadeh S, Maryam Gholinezhad-Chari M, Reza Ghadimi R. The effect of metformin treatment on the serum levels of homocysteine, folic acid, and vitamin B12 in patients with polycystic ovary syndrome. J Hum Reprod Sci. 2017;10(2):95–101. doi: 10.4103/jhrs.JHRS_74_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kumar A, Palfrey HA, Pathak R, Kadowitz PJ, Gettys TW, Murthy SN. The metabolism and significance of homocysteine in nutrition and health. Nutr Metab. 2017;14:78. doi: 10.1186/s12986-017-0233-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Forges T, Monnier-Barbarino P, Alberto JM, Guéant-Rodriguez RM, Daval JL, Guéant JL. Impact of folate and homocysteine metabolism on human reproductive health. Hum Reprod Update. 2007;13(3):225–238. doi: 10.1093/humupd/dml063. [DOI] [PubMed] [Google Scholar]
- 9.Berker B, Kaya C, Aytac R, Satıroglu H. Homocysteine concentrations in follicular fluid are associated with poor oocyte and embryo qualities in polycystic ovary syndrome patients undergoing assisted reproduction. Hum Reprod. 2009;24(9):2293–2302. doi: 10.1093/humrep/dep069. [DOI] [PubMed] [Google Scholar]
- 10.Chakraborty P, Goswami SK, Rajani S, Sharma S, Kabir SN, Chakravarty B, Jana K. Recurrent pregnancy loss in polycystic ovary syndrome: role of hyperhomocysteinemia and insulin resistance. PLoS One. 2013;8(5):e64446. doi: 10.1371/journal.pone.0064446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Glintborg D, Rubin KH, Nybo M, Abrahamsen B, Andersen M. Cardiovascular disease in a nationwide population of Danish women with polycystic ovary syndrome. Cardiovasc Diabetol. 2018;17:37. doi: 10.1186/s12933-018-0680-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fruzzetti F, Ghiadoni L, Virdis A, De Negri F, Perini D, Bucci F, Giannarelli C, Gadducci A, Taddei S. Adolescents with classical polycystic ovary syndrome have alterations in the surrogate markers of cardiovascular disease but not in the endothelial function. The possible benefits of metformin. Pediatr Adolesc Gynecol. 2016;29(5):489–495. doi: 10.1016/j.jpag.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Humphrey LL, Fu R, Rogers K, Freeman L, Helfand M. Homocysteine level and coronary heart disease incidence: a systematic review and meta-analysis. Mayo Clin Proc. 2008;83(11):1203–1212. doi: 10.4065/83.11.1203. [DOI] [PubMed] [Google Scholar]
- 14.Dattilo M, D’Amato G, Caroppo E, Menezo Y. Improvement of gamete quality by stimulating and feeding the endogenous antioxidant system: mechanisms, clinical results, insights on gene-environment interactions and the role of diet. J Assist Reprod Genet. 2016;33:1633–1648. doi: 10.1007/s10815-016-0767-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Botto L, Yang Q. 5,10-Methylenetetrahydrofolate reductase gene variants and congenital anomalies: a HuGE review. Am J Epiedemiol. 2000;151(9):862–877. doi: 10.1093/oxfordjournals.aje.a010290. [DOI] [PubMed] [Google Scholar]
- 16.Yang B, Liu Y, Li Y, Fan S, Zhi X, Lu X, Wang D, Zheng Q, Wang Y, Wang Y, Sun G. Geographical distribution of MTHFR C677T, A1298C and MTRR A66G Gene polymorphisms in China: findings from 15357 adults of han nationality. PLoS One. 2013;8(3):e57917. doi: 10.1371/journal.pone.0057917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.The Rotterdam ESHRE/ASRM-sponsored PCOS consensus workshop group Revised 2003 consensus on diagnostic criteria and longterm health risks related to polycystic ovary syndrome (PCOS) Hum Reprod. 2004;19(1):41–47. doi: 10.1093/humrep/deh098. [DOI] [PubMed] [Google Scholar]
- 18.Eskandari Z, Sadrkhanlou R-A, Nejati V, Tizro G. PCOS women show significantly higher homocysteine level, independent to glucose and E2 level. Int J Reprod BioMed. 2016;14(8):495–500. [PMC free article] [PubMed] [Google Scholar]
- 19.Asemi Z, Karamali M, Esmailzadeh A. Metabolic response to folate supplementation in overweight women with polycystic ovary syndrome: a randomized double-blind placebo-controlled clinical trial. Mol Nutr Food Res. 2014;58(7):1465–1473. doi: 10.1002/mnfr.201400033. [DOI] [PubMed] [Google Scholar]
- 20.Bailey SW, Ayling JE. The extremely slow and variable activity of dihydrofolate reductase in human liver and its implications for high folic acid intake. PNAS. 2009;106(36):15424–15429. doi: 10.1073/pnas.0902072106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cornet D, Clement A, Clement B, Menezo Y. High doses of folic acid induce a pseudo-methylenetetrahydrofolate syndrome. SAGE Open Med Case Rep. 2019;17(7):2050313X19850435.35. doi: 10.1177/2050313X19850435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Field MS, Stover PJ. Safety of folic acid. Ann N Y Acad Sci. 2018;1414:59–71. doi: 10.1111/nyas.13499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bailey SW, Ayling JE. The pharmacokinetic advantage of 5-methyltetrahydrofolate for minimization of the risk for birth defects. Sci Rep. 2018;8:4096. doi: 10.1038/s41598-018-22191-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kilicdag EB, Bagis T, Tarim E, Aslan E, Erkanli S, Simsek E, Haydardedeoglu B, Kuscu E. Administration of B-group vitamins reduces circulating homocysteine in polycystic ovarian syndrome patients treated with metformin: a randomized trial. Hum Reprod. 2005;20(6):1521–1528. doi: 10.1093/humrep/deh825. [DOI] [PubMed] [Google Scholar]
- 25.Pasquali R, Gambineri A. New perspectives on the definition and management of polycystic ovary syndrome. J Endocrinol Invest. 2018;41(10):1123–1135. doi: 10.1007/s40618-018-0832-1. [DOI] [PubMed] [Google Scholar]
- 26.Silvestris E, Cohen M, Cornet D, Jacquesson-Fournols L, Clement P, Chouteau J, Schneider M, Besnard T, Menezo Y. Supporting the one-carbon cycle restores ovarian reserve in subfertile women: absence of correlation with urinary bisphenol A concentration. BioResearch Open Access. 2017 doi: 10.1089/biores-2017.0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosenfield RL, Ehrmann DA. The pathogenesis of polycystic ovary syndrome (PCOS): the hypothesis of PCOS as functional ovarian hyperandrogenism revisited. Endocr Rev. 2016;37(5):467–520. doi: 10.1210/er.2015-1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deswal R, Yadav A, Dang AS. Sex hormone binding globulin - an important biomarker for predicting PCOS risk: a systematic review and meta-analysis. Syst Biol Reprod Med. 2018;64(1):12–24. doi: 10.1080/19396368.2017.1410591. [DOI] [PubMed] [Google Scholar]
- 29.Kajaia N, Binder H, Dittrich R, Oppelt PG, Flor B, Cupisti S, Beckmann MW, Mueller A. Low sex hormone-binding globulin as a predictive marker for insulin resistance in women with hyperandrogenic syndrome. Eur J Endocrinol. 2007;157:499–507. doi: 10.1530/EJE-07-0203. [DOI] [PubMed] [Google Scholar]
- 30.Jia L, Li J, He B, Jia Y, Niu Y, Wang C, Zhao R. Abnormally activated one-carbon metabolic pathway is associated with mtDNA hypermethylation and mitochondrial malfunction in the oocytes of polycystic gilt ovaries. Sci Rep. 2016;6:19436. doi: 10.1038/srep19436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vázquez-Martínez ER, Gómez-Viais YI, García-Gómez E, Reyes-Mayoral C, Reyes-Muñoz E, Camacho-Arroyo I, Cerbón M. DNA methylation in the pathogenesis of polycystic ovary syndrome. Reproduction. 2019;158:R27–R40. doi: 10.1530/REP-18-0449. [DOI] [PubMed] [Google Scholar]
- 32.Wierzbicki A. Homocysteine and cardiovascular disease: a review of the evidence. Diab Vasc Dis Res. 2007;4(2):143–150. doi: 10.3132/dvdr.2007.033. [DOI] [PubMed] [Google Scholar]
- 33.Studen KB, Pfeifer M. Cardiometabolic risk in polycystic ovary syndrome. Endocr Connect. 2018;7:R238–R251. doi: 10.1530/EC-18-0129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.James F, Toole JF, Malinow MR, Chambless LE, Spence JD, Pettigrew LC, Howard VJ, Sides EG, Wang CH, Stampfer M. Lowering homocysteine in patients with ischemic stroke to prevent recurrent stroke, myocardial infarction, and death. JAMA. 2004;291:565–575. doi: 10.1001/jama.291.5.565. [DOI] [PubMed] [Google Scholar]
- 35.Bønaa KH, Inger Njølstad I, Ueland PM, Schirmer H, Tverdal A, Steigen T, Wang H, Nordrehaug JE, Arnesen E, Rasmussen K, for the NORVIT Trial Investigators Homocysteine lowering and cardiovascular events after acute myocardial infarction. N Engl J Med. 2006;354(15):1578–1588. doi: 10.1056/NEJMoa055227. [DOI] [PubMed] [Google Scholar]
- 36.Zhang Q, Li S, Li L, Li Q, Ren K, Sun X, Li J. Metformin treatment and homocysteine: a systematic review and meta-analysis of randomized controlled trials. Nutrients. 2016;8(12):E798. doi: 10.3390/nu8120798. [DOI] [PMC free article] [PubMed] [Google Scholar]