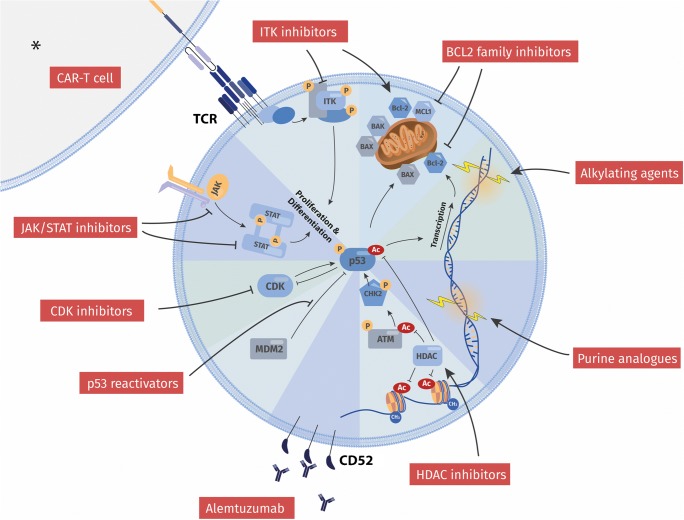
Fig. 1.
Overview of current and novel treatment strategies in T-PLL in relation to functional hallmarks of the tumor cell. Illustrated are categories of targets and modes of action of both established and most promising compound classes. Current treatment of T-PLL employs a (chemo)-immunotherapeutic approach based on alemtuzumab as well as on DNA-damaging alkylating agents and purine analogs. The inability of the T-PLL cell to evoke adequate p53-mediated responses to DNA-injuries, e.g., via enforced checkpoints or an apoptotic fate, stands at the center of its notorious therapy resistance. Novel strategies, however, revert the repressed state of functionally competent p53, or harness the apoptotically primed cellular state, or interrupt vital growth signal input. Respective examples are reactivation of p53 (e.g., via HDAC inhibitors, MDM2 inhibitors), targeting of BCL2 family members, inhibition of CDKs, or interception in JAK/STAT signal transduction. Efforts are also undertaken to utilize immunogenic cell death, e.g., by implementation of CAR-T cell therapies. * Generally, the role of cellular components of the tumor micromilieu is not established in T-PLL. Nevertheless, clinically proven GvL effects [48] and TCR-directed CAR-T cells in pre-clinical models [79••] implicate a therapeutic application of a T cell-mediated anti-T-PLL attack. Modulation of immune regulatory synapses, e.g., by perturbation of NK-cell tolerance (e.g., anti-KIR3DL2) or of macrophage inertia (e.g., SIRPαFc binding to CD47) represent interventional strategies of proven activity in other T cell tumors [76, 77], which have yet to be evaluated for their efficacy in T-PLL