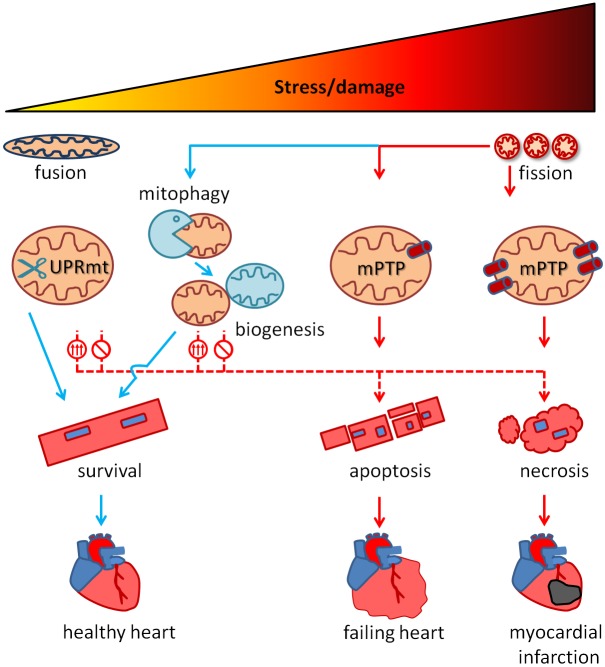
Figure 1.
Mitochondrial quality control (MQC) in heart disease. Everyday moderate stress/damage to mitochondria is repaired by MQC mechanisms (blue arrow), which prevent the occurrence of dysfunctional mitochondria that may exacerbate stress/damage. Less extensive damage is repaired by mitochondrial unfolded protein response (UPRmt), which properly folds misfolded proteins (chaperons) or cleaves them (proteases). Mitochondria that are beyond repair undergo mitophagy, which is tightly associated with mitochondrial biogenesis, serving to maintain a pool of healthy mitochondria. Mitochondrial fusion is beneficial as it reduces reactive oxygen species (ROS) generation. Mitochondrial fission promotes healthy phenotype when allowing mitophagy. Extreme stress beyond compensation by MQC systems induces mitochondrial permeability transition pore (mPTP) opening and cardiomyocytes death (solid red arrows). Less pronounced mPTP opening allows cell death by apoptosis, which is dominant in heart failure. The most extensive cell stress induces widespread mPTP opening in cardiomyocytes, which leads to necrotic cell death, a predominant mechanism in acute ischemia-reperfusion, ie, myocardial infarction. Extremely high cell stresses are linked to mitochondrial fission, which exacerbates ROS generation. Inadequate MQC system activation induces cell death even during exposure to less extensive cell stress (red dotted arrows). This includes excessive activation of either UPRmt, mitophagy, or mitochondrial fission, which cause further dysfunction of mitochondria, or insufficient activation of MQC mechanisms that do not repair/eliminate dysfunctional mitochondria.