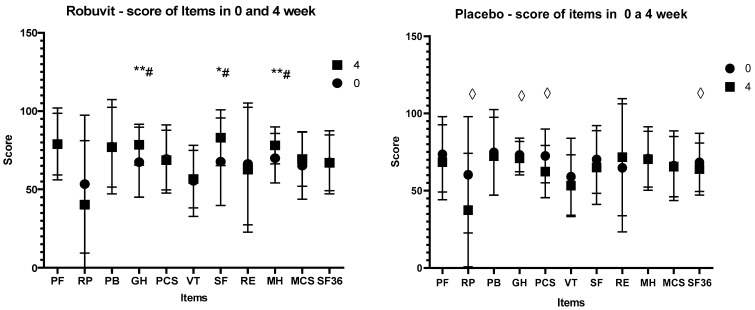
Figure 3.
Changes in scores of individual health items after 4–week Robuvit and placebo administration (numbers of analysed data in both groups are given in Flow diagram). R—Robuvit, Pl—placebo, PF—physical functioning, RP—role limitations due to physical health, PB—pain, GH—general health, PCS—physical Component Summary, VT—vitality, SF—social functioning, RE—role limitations due to emotional problems, MH—mental health, MCS—mental component Summary, SF–36, whole physical and mental health. * p < 0.05 for positive difference in Robuvit group between week 4 and week 0, ** p < 0.01 for positive difference in Robuvit group between week 4 and week 0, ◊ p < 0.05 for negative difference in placebo group between week 4 and week 0, # p < 0.05 for difference between Robuvit and placebo groups.