Figure 2.
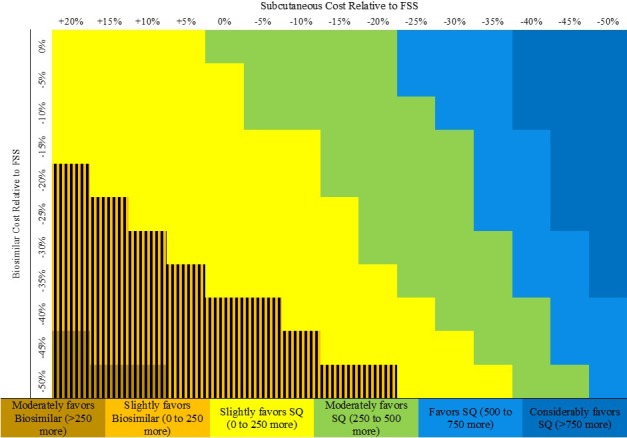
Multiway sensitivity analysis evaluating the relative impact of varying biosimilar rituximab cost vs subcutaneous (SQ) rituximab cost on annual infusion center capacity to administer subsequent rituximab doses. We assumed that the infusion center has a fixed budget of $5 321 950 (includes costs of drug, nondrug consumables, personnel, and space) and evaluated how varying these costs would affect the number of second rituximab doses that can be provided in 1 year. All drug cost savings are relative to the Federal Supply Schedule (FSS) program costs of originator intravenous rituximab. Subcutaneous rituximab requires 30 minutes to administer. The number reflected by the color of each cell represents this difference: number of treatments if subcutaneous rituximab is used − number of treatments if biosimilar rituximab is used. The hatched area reflects the threshold across which biosimilar rituximab is associated with greater capacity when compared with subcutaneous rituximab.
