Abstract
Objective
A global pandemic caused by a novel coronavirus (Covid-19) has created unique challenges to providing timely care for cancer patients. In early-stage cervical cancer, postponing hysterectomy for 6–8 weeks is suggested as a possible option in the Covid-19 burdened hospitals. Yet, literature examining the impact of surgery wait-time on survival in early-stage cervical cancer remains scarce. This study examined the association between surgery wait-time of 8 weeks and oncologic outcome in women with early-stage cervical cancer.
Methods
This is a single institution retrospective observational study at a tertiary referral medical center examining women who underwent primary hysterectomy or trachelectomy for clinical stage IA-IIA invasive cervical cancer between 2000 and 2017 (N = 217). Wait-time from the diagnosis of invasive cervical cancer via biopsy to definitive surgery was categorized as: short wait-time (<8 weeks; n = 110) versus long wait-time (≥8 weeks; n = 107). Propensity score inverse probability of treatment weighting was used to balance the measured demographics between the two groups, and disease-free survival (DFS) and overall survival (OS) were assessed. A systematic literature review with meta-analysis was additionally performed.
Results
In a weighted model (median follow-up, 4.6 years), women in the long wait-time group had DFS (4.5-year rates, 91.2% versus 90.7%, hazard ratio [HR] 1.11, 95% confidence interval [CI] 0.47–2.59, P = 0.818) and OS (95.0% versus 97.4%, HR 1.47, 95%CI 0.50–4.31, P = 0.487) similar to those in the short wait-time group. Three studies were examined for meta-analysis, and a pooled HR for surgery wait-time of ≥8 weeks on DFS was 0.96 (95%CI 0.59–1.55).
Conclusion
Our study suggests that wait-time of 8 weeks for hysterectomy may not be associated with short-term disease recurrence in women with early-stage cervical cancer.
Keywords: Cervical cancer, Early stage, Wait time, Surgery, Hysterectomy, Survival
Highlights
-
•
Examined effect of long wait-time (≥8 weeks) for surgical treatment on survival of women with early-stage cervical cancer.
-
•
Long surgery wait-time was not associated with short-term disease recurrence of women with early-stage cervical cancer.
-
•
Systematic review suggests that surgery wait-time for up to 8 weeks may have limited/modest effect on disease recurrence.
1. Introduction
Cervical cancer is the fourth most common cancer in women worldwide [1]. In the United States, it is estimated that there will be 13,800 new cases and 4290 deaths from cervical cancer in 2020 [2]. Treatment of women with cervical cancer is largely dependent on the stage of cancer [3]. Hysterectomy-based approach is the surgical treatment of choice for women with early-stage cervical cancer [3]. In the United States, the vast majority of women with early-stage cervical cancer receive hysterectomy (Supplemental Fig. S1). For instance, 82.7% of women with stage T1b(≤4 cm) disease underwent hysterectomy-based surgical treatment from 2010 to 2016 in the United States.
In the past few months, a global pandemic caused by a novel coronavirus infection (Covid-19) has led to a large number of deaths, creating unique challenges to providing timely care for cancer patients [[4], [5], [6], [7], [8]]. Mounting evidence shows that cancer patients are at higher risk for severe illness from Covid-19. For this reason, extra care and caution will be needed when proceeding with surgical treatment for patients with malignancy [9]. An expert panel suggested postponing hysterectomy for 6 to 8 weeks as a possible option for early-stage cervical cancer in a Covid-19 hotspot area [10]. This suggestion is particularly applicable for patients with stage IA2-IIA1 cervical cancer because radical hysterectomy for these cancer stages is likely via laparotomy requiring inpatient hospitalization [11]. At centers heavily affected by this pandemic, inpatient resources may be limited and patients may be at risk of Covid-19 acquisition during hospitalization.
Prior studies mainly examined the survival effect of wait-time for definitive radiotherapy [[12], [13], [14], [15], [16], [17], [18], [19], [20], [21]], and evidence examining the impact of wait-time for hysterectomy on survival in early cervical cancer remains scarce [16,17]. Moreover, no supporting evidence was provided in the aforementioned expert panel suggestion [10]. The objective of the current study was to examine the significance of wait-time from cervical biopsy to surgical treatment on survival of women with early-stage cervical cancer. Specifically, this study assessed the cutoff of 8 weeks per the recent expert panel recommendation [10].
2. Materials and methods
2.1. Data source and eligibility
This is a single institution retrospective observational study at a tertiary referral medical center. After Institutional Review Board approval was obtained at the University of Southern California, a previously organized institutional database was queried to identify consecutive women who underwent primary hysterectomy or trachelectomy for clinical stage IA-IIA invasive cervical cancer at the Los Angeles County Medical Center from 2000 to 2017 [22,23]. Those who had pre-invasive cervical cancer at biopsy, did not have hysterectomy or trachelectomy, and had no information for wait-time were excluded from the study.
2.2. Clinical information
Among those who were eligible for the study, patient demographics, laboratory test results, tumor characteristics, treatment types, and survival outcomes were collected from archived medical records. Patient demographics included age at diagnosis, year at diagnosis, race/ethnicity, parity, body mass index, and cigarette use. Laboratory test results at time of diagnosis included white blood cell counts, hemoglobin, platelet, bicarbonate, blood urea nitrogen, creatinine, and albumin levels. Tumor characteristics included histology types, pretreatment clinical cancer stage reclassified per the FIGO 2018 classification at diagnosis [24], and presence of pelvic or para-aortic lymph node metastasis per surgical pathological testing. Treatment types included surgery type and use of postoperative radiotherapy.
2.3. Study definition
Wait-time was defined as the time interval between the cervical cancer diagnosis with biopsy and surgical treatment with hysterectomy or trachelectomy. Patients were divided into two groups based on wait-time from the diagnosis of invasive cervical cancer via biopsy to definitive surgery: short wait-time (<8 weeks) versus long wait-time (≥8 weeks) as suggested by a recent expert panel [10]. Disease-free survival (DFS) was defined as the time interval between surgical treatment for cervical cancer and the first recurrence of cervical cancer or death due to cervical cancer. Overall survival (OS) was defined as the time interval between surgery for cervical cancer and death from any cause (all-cause). In this analysis, surgery date rather than diagnosis date was used as the starting point of survival follow-up in order to standardize the exposure effect of wait-time.
2.4. Statistical consideration
Propensity score inverse probability of treatment weighting was used to balance the demographics between the two groups, as described previously (Fig. S2) [25,26], and survival outcome measures for DFS and OS were assessed. Due to the small sample size, size effect was used for the selection of covariates, and all the covariates with standardized difference [SD] ≥0.20 were entered in the model as described previously [27]. In the propensity score weighted model, women with long wait-time were assigned a weight of 1/(propensity score) whereas those with short wait-time were assigned a weight of 1/(1-propensity score) [26]. Stabilized weights were used and threshold technique was used at the 1st and 99th percentile of the weight distribution [26,28].
In a propensity score weighted model, size effect was assessed with SD and a value of >0.20 was considered presence of size effect with clinical imbalance [29]. Kaplan-Meier method was used to construct survival curves, and a Cox proportional hazard regression model was fitted to estimate effect size of wait-time on survival outcome, expressed with hazard ratio (HR) and 95% confidence interval (CI). Proportional hazard assumption was tested and satisfied without interaction to time.
Various sensitivity analyses were undertaken to examine the robustness of the study findings. First, analysis was restricted to cases with higher stage disease (FIGO stage IB-IIA disease). Second, generalized boosted model, a class of machine learning, was fitted to balance the two group and estimate the weights. Third, cases were restricted to squamous tumors as this is the most common histology type in cervical cancer. Last, wait-time was examined as a continuous variable, adjusting for a priori prognostic factors.
All statistical analyses were based on two-tailed hypothesis, and a P < 0.05 was considered statistically significant. Statistical Package for Social Sciences (version 25.0, Armonk, NY, USA) and R version 3.5.3 (R Foundation for Statistical Computing, Vienna, Austria) were used for analyses. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines were used to outline the results of this observational cohort study [30].
2.5. Systematic review of literature and meta-analysis
A systematic review of the literature was undertaken to assess the survival impact of wait-time for hysterectomy in early-stage cervical cancer. The detailed methodology is described in Supplemental Methods S1. In brief, multiple public search engines, PubMed, Scopus, and Cochrane Central Register of Controlled Trials (CENTRAL) were utilized and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines were consulted to identify the eligible studies [31]. Studies with pregnancy cases were not included in the analysis. Among evaluated studies, meta-analysis was performed to estimate effect size of long wait-time and oncologic outcome (DFS and OS).
3. Results
There were 230 women with stage IA-IIA cervical cancer identified during the study period. Of those, 13 women were excluded (11 women with pre-invasive disease and 2 women without wait-time information), and the remaining 217 women represented the study population. The median wait-time for the whole cohort was 55 (IQR 41–82) days; 110 (50.7%) women had a short wait-time and 107 (49.3%) had a long wait-time. The group-specific median wait-time was 41 (IQR 29–49) days for the short wait-time group and 82 (IQR 66–119) days for the long wait-time group, respectively.
Patient demographics are shown in Table 1 . In this cohort, the median age was 45 years, and the most common histology was squamous (n = 148, 68.2%). The two most common cancer stages were IB1 (n = 64, 29.5%) and IA1 (n = 63, 29.0%), followed by IB2 (n = 55, 25.3%). Among the measured variables, cancer stage was the only preoperative factor associated with wait-time. Specifically, higher stage was associated with shorter wait-time among stage T1 disease (79, 65, 52, 49, and 42 days for stage IA1, IA2, IB1, IB2, and IB3 respectively, absolute difference 37 days; P < 0.001).
Table 1.
Patient demographics (N = 217).
| Characteristic |
Short wait-time |
Long wait-time |
P-value |
|---|---|---|---|
| Number | n = 110 | n = 107 | |
| Age | 47.5 (10.8) | 46.7 (11.6) | 0.463 |
| <30 | 6 (5.5%) | 3 (2.8%) | |
| 30–39 | 24 (21.8%) | 29 (27.1%) | |
| 40–49 | 37 (33.6%) | 42 (39.3%) | |
| 50–59 | 26 (23.6%) | 17 (15.9%) | |
| 60–69 | 15 (13.6%) | 12 (11.2%) | |
| ≥70 | 2 (1.8%) | 4 (3.7%) | |
| Year | 0.709 | ||
| 2000–2005 | 25 (22.7%) | 20 (18.7%) | |
| 2006–2011 | 59 (53.6%) | 58 (54.2%) | |
| 2012–2017 | 26 (23.6%) | 29 (27.1%) | |
| Race/ethnicity | 0.617 | ||
| White | 10 (9.1%) | 7 (6.5%) | |
| Black | 5 (4.5%) | 4 (3.7%) | |
| Hispanic | 77 (70.0%) | 80 (74.8%) | |
| Asian | 11 (10.0%) | 6 (5.6%) | |
| Others* | 7 (6.4%) | 10 (9.3%) | |
| Parity | 0.972 | ||
| Nullipara | 7 (6.4%) | 6 (5.6%) | |
| Multipara | 97 (88.2%) | 95 (88.8%) | |
| Unknown | 6 (5.5%) | 6 (5.6%) | |
| Body habitus** | 0.358 | ||
| Normal/underweight | 27 (24.5%) | 16 (15.0%) | |
| Overweight | 35 (31.8%) | 30 (28.0%) | |
| Class I | 26 (23.6%) | 28 (26.2%) | |
| Class II | 5 (4.5%) | 8 (7.5%) | |
| Class III | 6 (5.5%) | 8 (7.5%) | |
| Unknown | 11 (10.0%) | 17 (15.9%) | |
| Cigarette use | 0.924 | ||
| No | 92 (83.6%) | 88 (82.2%) | |
| Yes | 14 (12.7%) | 14 (13.1%) | |
| Unknown | 4 (3.6%) | 5 (4.7%) | |
| Histology | 0.178 | ||
| Squamous | 72 (65.5%) | 76 (71.0%) | |
| Adenocarcinoma | 32 (29.1%) | 22 (20.6%) | |
| Adenosquamous | 6 (5.5%) | 6 (5.6%) | |
| Others | 0 | 3 (2.8%) | |
| Clinical stage | <0.001 | ||
| IA1 | 20 (18.2%) | 43 (40.2%) | |
| IA2 | 8 (7.3%) | 13 (12.1%) | |
| IB1 | 36 (32.7%) | 28 (26.2%) | |
| IB2 | 39 (35.5%) | 16 (15.0%) | |
| IB3 | 6 (5.5%) | 3 (2.8%) | |
| IIA | 1 (0.9%) | 4 (3.7%) | |
| Pelvic nodal mets | <0.001 | ||
| No | 81 (73.6%) | 61 (57.0%) | |
| Yes | 15 (13.6%) | 6 (5.6%) | |
| Not sampled | 14 (12.7%) | 40 (37.4%) | |
| Para-aortic nodal mets | 0.679 | ||
| No | 15 (13.6%) | 11 (10.3%) | |
| Yes | 2 (1.8%) | 3 (2.8%) | |
| Not sampled | 93 (84.5%) | 93 (86.9%) | |
| Surgery type | 0.004 | ||
| Abdominal RH† | 80 (72.7%) | 52 (48.6%) | |
| LSC RH | 6 (5.5%) | 8 (7.5%) | |
| RA-RH | 1 (0.9%) | 4 (3.7%) | |
| TAH | 10 (9.1%) | 21 (19.6%) | |
| TLH | 4 (3.6%) | 15 (14.0%) | |
| Vaginal | 6 (5.5%) | 3 (2.8%) | |
| Trachelectomy | 1 (0.9%) | 3 (2.8%) | |
| Unknown | 2 (1.8%) | 1 (0.9%) | |
| Adnexectomy | 0.718 | ||
| No | 43 (39.1%) | 39 (36.4%) | |
| Yes | 64 (58.2%) | 63 (58.9%) | |
| Unknown | 3 (2.7%) | 5 (4.7%) | |
| Postop radiotherapy | 0.007 | ||
| No | 75 (68.2%) | 90 (84.1%) | |
| Yes | 35 (31.8%) | 17 (15.9%) | |
| WBC | 7.8 (2.2) | 7.8 (2.2) | 0.780 |
| <10 | 92 (83.6%) | 93 (86.9%) | |
| ≥10 | 17 (15.5%) | 13 (12.1%) | |
| Unknown | 1 (0.9%) | 1 (0.9%) | |
| Hemoglobin | 12.9 (1.5) | 12.6 (1.8) | 0.949 |
| ≥10 | 102 (92.7%) | 98 (91.6%) | |
| <10 | 7 (6.4%) | 8 (7.5%) | |
| Unknown | 1 (0.9%) | 1 (0.9%) | |
| Platelet | 272 (74) | 288 (78) | 0.114 |
| <400 | 104 (94.5%) | 98 (91.6%) | |
| ≥400 | 5 (4.5%) | 8 (7.5%) | |
| Unknown | 1 (0.9%) | 1 (0.9%) | |
| HCO3 | 25.3 (2.9) | 24.8 (2.9) | 0.994 |
| <23 | 93 (84.5%) | 91 (85.0%) | |
| ≥23 | 16 (14.5%) | 15 (14.0%) | |
| Unknown | 1 (0.9%) | 1 (0.9%) | |
| BUN | 12.0 (3.8) | 12.3 (4.8) | 0.748 |
| ≤20 | 106 (96.4%) | 101 (94.4%) | |
| >20 | 3 (2.7%) | 5 (4.7%) | |
| Unknown | 1 (0.9%) | 1 (0.9%) | |
| Creatinine | 0.6 (0.1) | 0.6 (0.1) | 0.832 |
| ≤1.0 | 108 (98.2%) | 104 (97.2%) | |
| >1.0 | 1 (0.9%) | 1 (0.9%) | |
| Unknown | 1 (0.9%) | 2 (1.9%) | |
| Albumin | 4.3 (0.4) | 4.3 (0.4) | 0.996 |
| ≥4.0 | 92 (83.6%) | 89 (83.2%) | |
| <4.0 | 17 (15.5%) | 17 (15.9%) | |
| Unknown | 1 (0.9%) | 1 (0.9%) |
Mean (standard deviation) or number (percentage per group) is shown. Univariable analysis for P-value. *including unknown. **Per the CDC classification. †including type II. Abbreviations: RH, radical hysterectomy; LSC, laparoscopic; RA, robotic-assisted; TAH, total abdominal hysterectomy; mets, metastasis; TLH, total laparoscopic hysterectomy; and postop, postoperative.
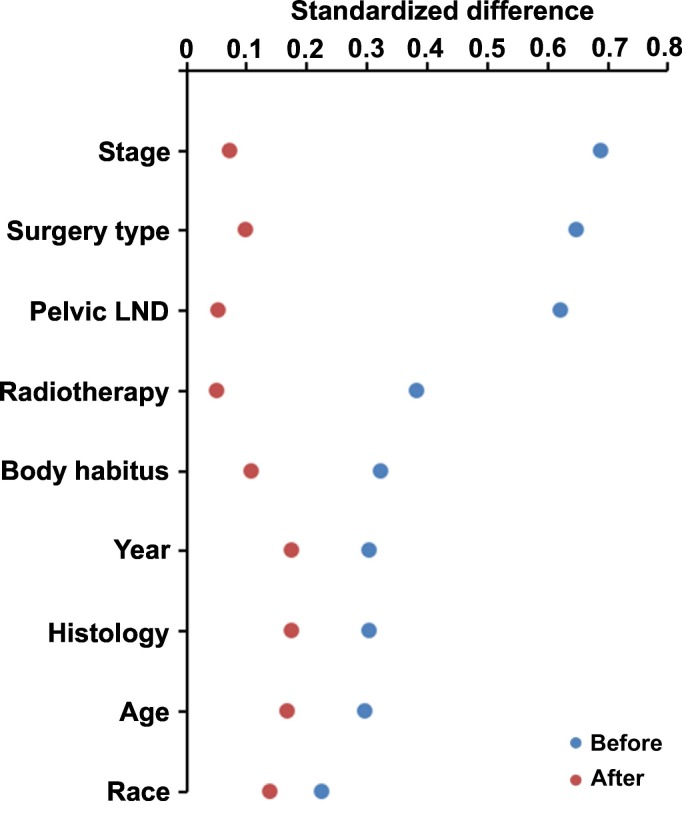
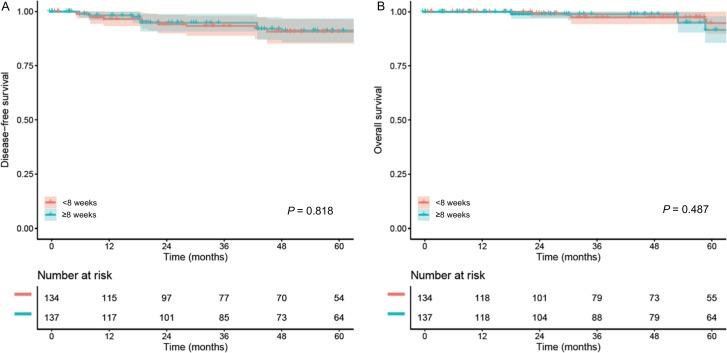
In a propensity score weighted model (n = 272), the measured covariates were overall balanced without clinical imbalance between the two groups (all, SD ≤ 0.20; Fig. 1 and Table S1). The median follow-up of the weighted model was 4.6 years, with 21 (7.8%) recurrences and 14 (5.1%) deaths during follow-up. Women in the long wait-time group had DFS (4.5-year rates 91.2% versus 90.7%, HR 1.11, 95%CI 0.47–2.59, P = 0.818; Fig. 2A) and OS (4.5-year rates 95.0% versus 97.4%, HR 1.47, 95%CI 0.50–4.31, P = 0.487; Fig. 2B) similar to those in the short wait-time group. This association was unchanged when the whole cohort was analyzed with a generalized boosted model (data not shown).
Fig. 1.
Balance statistics with standardized difference for PS-IPTW.
Standardized differences before and after PS-IPTW are shown: the value of >0.2 indicates presence of size effect for clinical imbalance between the two groups. Abbreviations: PS-IPTW, propensity score inverse probability of treatment weighting; LND, lymphadenectomy.
Fig. 2.
Survival outcomes based on wait-time for surgery in the whole cohort (PS-IPTW model).
Disease-free survival (panel A) and overall survival (panel B) are shown based on wait-time from cervical cancer diagnosis to hysterectomy or trachelectomy. Cox proportional hazard regression model for P-values. Color bands indicate 95% confidence interval.
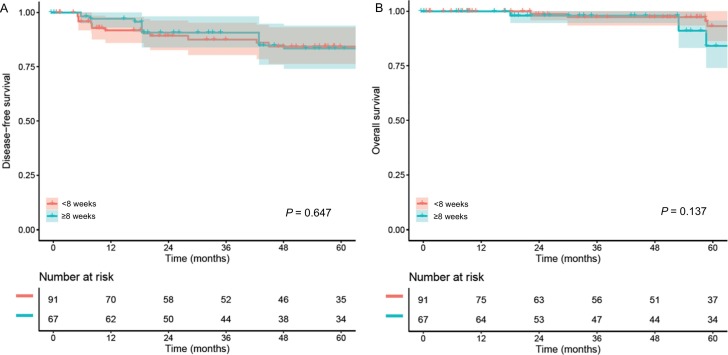
In a sensitivity analysis, cases were limited to stage IB-IIA disease. In a propensity score weighted model (n = 158), there were 24 (15.2%) recurrences and 14 (8.9%) deaths during the follow-up (median, 4.8 years). Similar to the whole cohort, long wait-time was statistically not associated with DFS (4.5-year rates 83.4% versus 84.3%, HR 1.21, 95%CI 0.54–2.70, P = 0.647; Fig. 3A) and OS (4.5-year rates 91.1% versus 97.4%, HR 2.40, 95%CI 0.75–7.61, P = 0.137; Fig. 3B) when compared to the short wait-time in a propensity score weighted model.
Fig. 3.
Survival outcomes based on wait-time for surgery in stage IB-IIA disease (PS-IPTW model).
Disease-free survival (panel A) and overall survival (panel B) are shown based on wait-time from cervical cancer diagnosis to hysterectomy or trachelectomy. Cox proportional hazard regression model for P-values. Color bands indicate 95% confidence interval.
Among squamous tumors (n = 186) in a propensity score weighted model, there were 16 (8.6%) recurrences and 13 (7.0%) deaths recorded during follow-up (median, 4.4 years). DFS (4.5-year rates 85.6% versus 92.3%, HR 1.67, 95%CI 0.61–4.56, P = 0.318; Fig. S3A) and OS (4.5-year rates 90.0% versus 97.8%, HR 2.30, 95%CI 0.67–7.98, P = 0.187; Fig. S3B) were statistically similar between the long wait-time group and the short wait-time group.
Moreover, when wait-time was examined as a continuous variable, there was no association with DFS (adjusted-HR per one additional wait day 0.99, 95%CI 0.97–1.01, P = 0.290) and overall survival (adjusted-HR per one additional wait day 0.99, 95%CI 0.98–1.01, P = 0.166). After controlling for age, stage, histology, nodal status, and postoperative therapy, this association remained unchanged (data not shown).
4. Discussion
Key findings of the current study are that nearly half of our study patients had a surgical wait-time of approximately two months and wait-time of 8 weeks for hysterectomy was not associated with decreased oncologic outcome in the short-term. Several areas deserve further discussion.
In April 2020, the number of Covid-19 cases and related deaths is continuing to increase in the United States [32]. Some of the most Covid-19 burdened areas in the United States are high incidence areas of cervical cancer [33,34]. Thus, it is likely that there are a number of patients in whom hysterectomy for early cervical cancer is being postponed for a variety of reasons, such as hospital restriction, patient's infection, or surgeon's choice. As delay in surgery would most likely cause anxiety in both patient and care providers, data examining the outcome of long wait-time for cervical cancer surgery is of utmost importance in the current Covid-19 pandemic.
A systematic literature review found that evidence examining the association between surgery wait-time and oncologic outcome has been fairly limited (Fig. S4). There were only three studies that met the study searching criteria, including the current study, and the sample size of these studies were relatively small (median 217 cases, range 117–441; Table 2 ) [16,17]. In a retrospective cohort study of 117 patients diagnosed with IA-IIA cervical cancer at a single institution in Japan, with the median follow-up of 4.3 years, longer wait-time ≥ 50 days to surgery was not associated with DFS or OS [17].
Table 2.
Wait-time for surgical treatment and oncologic outcome (systematic review).
| Author | Umezu [17] | Nanthamongkolkul [16] | Matsuo* |
|---|---|---|---|
| Country | Japan | Thailand | United States |
| Year | 2012 | 2015 | 2020 |
| No. | n = 117 | n = 441 | n = 217 |
| Stage | IA-IIA (FIGO 2009) | IA2-IB1 (FIGO 2009) | IA-IIA (FIGO 2018) |
| Treatment | Surgery | Surgery | Surgery |
| Population | NA | NA | Hispanic (72.4%) |
| Median wait-time | 48 days | 43 days | 55 days |
| Cutoff for wait-time | 50 days | 56 days | 56 days |
| Median follow-up | 4.3 years | 4.2 years | 4.6 years |
| DFS | |||
| HR (95%CI) | NA | 0.9 (0.5–1.6) | 1.11 (0.47–2.59) |
| 5-yr rates | 80.9% vs 91.4% | 89.6% vs 86.8% | 91.2% vs 90.7%** |
| P-value | P = 0.106 | P = 0.677 | P = 0.818 |
| OS | |||
| HR (95%CI) | NA | NA† | 1.47 (0.50–4.31) |
| 5-yr rates | 92.5% vs 96.7% | 96.0% vs 90.7% | 95.0% vs 97.4%** |
| P-value | P = 0.653 | P = 0.973 | P = 0.487 |
Wait-time is shown in days. Survival rates represent long wait-time versus short wait-time. † 5-year OS rates were similar (P = 0.973) but the authors reported the results of time-varying analysis demonstrating that waiting-time ≥ 56 days was associated with improved OS before 5 years (HR 0.4, 95%CI 0.1–1.3) and decreased OS after 5 years (HR 3.4, 95%CI 1.3–9.2). *Current study. **4.5-year survival rates. Abbreviations: HR, hazard ratio; CI, confidence interval, FIGO, International Federation of Gynecology and Obstetrics; CRT, concurrent chemoradiotherapy; NA, not available; yr, year; DFS, disease-free survival; and OS, overall survival.
Another retrospective study from Thailand examining 441 cases of stage IA2-IB1 cervical cancer showed that 5-year DFS (89.6% versus 86.8%) and OS (96.0% versus 90.7%) rates were statistically similar between wait-time of ≥8 versus < 8 weeks [16]. Notably, the survival curves crossed over at around 8 years, and a time-varying analysis showed that those in the long wait-time group had poorer OS versus the short wait-time group after 5 years. As the median follow-up of their study was short (4.2 years), the interpretation of long follow-up is limited. Last, our study found that with the median follow-up of 4.6 years, longer wait-time ≥ 8 weeks to surgery was not associated with decreased survival in women with clinical stage IA-IIA cervical cancer.
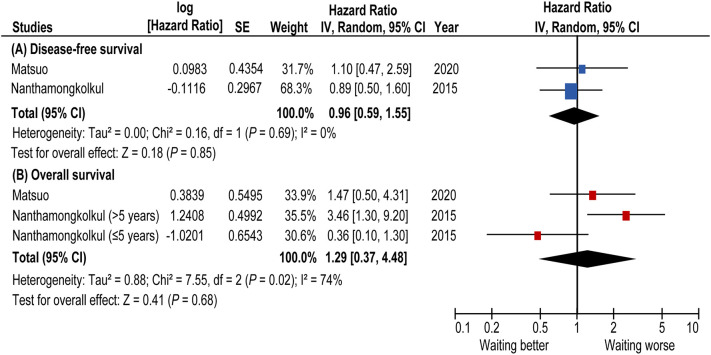
Collectively, the Thailand and U.S. studies were evaluable for statistical output to estimate the effect size of long-wait time for survival outcome (Fig. 4 ). The available data from the two studies suggest a limited/modest effect of longer wait-time for surgery on survival. A pooled HR of surgery wait-time of ≥8 weeks for DFS was 0.96 (95%CI 0.59–1.55). In an exploratory analysis, effect size for OS was estimated by combining HR from our study and two time-varying HRs from the Thailand study as their study did not provide overall HR for OS [16]. A pooled HR for OS was 1.29 (95%CI 0.37–4.48) but the wide range of 95%CI dilutes the interpretation of results, necessitating further studies to examine this association.
Fig. 4.
Forest plots for survival estimates (systematic review).
Forest plots for (A) DFS (B) OS are shown. In an exploratory analysis, two time-varying HRs from one study [16] were analyzed as discrete values. A forest plot from a random effect analysis of two studies including our study stratified by inclusion criteria of systematic review. Centers of squares and horizontal bars through each indicate point and 95% confidence interval estimates of individual study hazard ratio. Area of squares indicates the relative weights of the individual studies. Moderate heterogeneity (I2 = 74%) was observed in OS analysis and no heterogeneity (I2 = 0%) was observed in DFS analysis. Some values listed above might be slightly different from the original values because of calculating by Revman5.3™. Abbreviations: DFS, disease-free survival; and CI, confidence interval.
A rationalized hypothesis to explain our study results is that cervical cancer tumors may grow slowly when disease is in an early stage, and waiting for surgical treatment for a short period of time may not impact oncologic outcome in early disease. This is based on the observation that disease progression is rare among women with pregnancy complicated by early-stage cervical cancer, and those affected patients often undergo expectant delay in delivery for the purpose of fetal maturation during pregnancy without compromising survival outcome [[35], [36], [37], [38], [39], [40], [41]].
The median wait-time for surgical treatment in early cervical cancer ranged from 43 to 55 days among the published literature including our study (Table 2). This means that nearly half of patients with early cervical cancer wait more than two months to receive surgical treatment in real-world practice. Of note, this median wait-time for hysterectomy is similar to the 6–8 week time-frame suggested by the expert panel for postponing surgery during the Covid-19 pandemic [10]. It is therefore speculated that surgery wait-time of 6–8 weeks may not be considered a “prolonged” wait-time period for early cervical cancer.
However, this data needs to be interpreted with caution in the case of higher stage disease. In our analysis, albeit statistically non-significant, survival probability and hazard risk for OS in stage IB-IIA diseases was pointing towards a negative impact with long wait-time for surgery (5-year OS rate 91.1% versus 97.4%; HR 2.40, 95%CI 0.75–7.61). The wide range of confidence interval clearly implies that sample size and event number were both limited to detect statistically significant difference. Moreover, the number of patients with stage IB disease with tumor size 2-4 cm was limited for sensitivity analysis on this subcohort. With α-level of 0.05, our study was underpowered for OS in stage IB-IIA disease (<80%).
Our study also found that, of the measured variables, cancer stage was the only preoperative factor associated with wait-time. Specifically, patients with higher clinical cancer stage tended to have shorter wait-time from diagnosis to surgery, and there was approximately a five-week difference in wait-time between stage IA and IB3 disease in our study. This is similar to the findings in other studies, in which patients with shorter wait-times tended to have larger and higher-stage tumors [13,14].
Strengths of this study include that a statistical analysis with propensity score inverse probability of treatment weighting enriched the statistical rigor. Additional sensitivity analysis and systematic literature review with meta-analysis enhanced the robustness of study findings. A limitation of the study includes that this is a retrospective study that may have an unmeasured bias. For example, the exact reason for delay from diagnosis to surgery is not retrievable from the archived records. Performance status, medical condition, and socio-economic reasons may have affected the wait-time as well as the survival outcome, and missing these factors limit the interpretation of the study. It is also possible that longer follow-up would elucidate differences in OS, as a prior study found differences in outcomes only after 5 years of follow-up [16]. Most importantly, the sample size of our study is somehow limited raising the potential for type II error as described earlier.
In conclusion, our study suggests that a wait-time of 8 weeks between diagnosis and definitive surgical treatment with hysterectomy or trachelectomy may not increase risk of short-term disease recurrence in women with early-stage cervical cancer. While this information partly supports the recent suggestion to consider postponement of surgery for 6–8 weeks for the management of early cervical cancer during the Covid-19 pandemic, further studies with a larger sample size are warranted to examine the effect of long wait-time on survival in stage IB-IIA cervical cancer, particularly for tumor size >2 cm.
Last, while most of women with early-stage cervical cancer undergo surgical treatment in the United States (Supplemental Fig. S1), consideration of non-surgical approaches with definitive chemo-radiotherapy may also need to be considered if the Covid-19 crisis is sustained [42]. A prior phase III clinical trial showed a similar survival outcome between an upfront surgical approach and definitive radiotherapy for early-stage cervical cancer [43].
Author contributions
Conceptualization: K.M.; Data curation: M.S.H., A.V.C., S.M., K.M.; Formal analysis: K.M., S.M., D.J.N.; Funding acquisition: K.M., L.D.R.; Investigation: all authors; Methodology: K.M., S.M.; Project administration: K.M.; Resources: K.M.; Software: K.M., S.M., D.J.N.; Supervision: K.M.; Validation: K.M., S.M.; Visualization: K.M., S.M.; Writing - original draft: H.N., K.M.; Writing - review & editing: all authors.
Funding source
Ensign Endowment for Gynecologic Cancer Research (K.M.)
Declaration of competing interest
Consultant, Quantgene (L.D.R.); honorarium, Chugai, textbook editorial expense, Springer, and investigator meeting attendance expense, VBL therapeutics (K.M.); research grant, MSD (S.M.); none for others.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ygyno.2020.05.019.
Appendix A. Supplementary data
Supplementary material
References
- 1.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2020. CA Cancer J. Clin. 2020;70:7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 3.Cervical cancer. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) http://www.nccn.org
- 4.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., Zhao X., Huang B., Shi W., Lu R., Niu P., Zhan F., Ma X., Wang D., Xu W., Wu G., Gao G.F., Tan W., China Novel Coronavirus I., Research T. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li Q., Guan X., Wu P., Wang X., Zhou L., Tong Y., Ren R., Leung K.S.M., Lau E.H.Y., Wong J.Y., Xing X., Xiang N., Wu Y., Li C., Chen Q., Li D., Liu T., Zhao J., Liu M., Tu W., Chen C., Jin L., Yang R., Wang Q., Zhou S., Wang R., Liu H., Luo Y., Liu Y., Shao G., Li H., Tao Z., Yang Y., Deng Z., Liu B., Ma Z., Zhang Y., Shi G., Lam T.T.Y., Wu J.T., Gao G.F., Cowling B.J., Yang B., Leung G.M., Feng Z. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N. Engl. J. Med. 2020;382:1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang C., Horby P.W., Hayden F.G., Gao G.F. A novel coronavirus outbreak of global health concern. Lancet. 2020;395:470–473. doi: 10.1016/S0140-6736(20)30185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., Wang B., Xiang H., Cheng Z., Xiong Y., Zhao Y., Li Y., Wang X., Peng Z. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020 doi: 10.1001/jama.2020.1585. in-press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yu J., Ouyang W., Chua M.L.K., Xie C. SARS-CoV-2 transmission in patients with cancer at a tertiary care hospital in Wuhan, China. JAMA Oncol. 2020 doi: 10.1001/jamaoncol.2020.0980. in-press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dowdy S, Fader AN. Surgical considerations for gynecologic oncologists during the COVID-19 pandemic. Society of Gynecologic Oncology (accessed April 14, 2020).
- 10.Ramirez P.T., Chiva L., Eriksson A.G.Z., Frumovitz M., Fagotti A., Gonzalez Martin A., Jhingran A., Pareja R. COVID-19 global pandemic: options for management of gynecologic cancers. Int J Gynecol Cancer. 2020 doi: 10.1136/ijgc-2020-001419. in-press. [DOI] [PubMed] [Google Scholar]
- 11.Ramirez P.T., Frumovitz M., Pareja R., Lopez A., Vieira M., Ribeiro R., Buda A., Yan X., Shuzhong Y., Chetty N., Isla D., Tamura M., Zhu T., Robledo K.P., Gebski V., Asher R., Behan V., Nicklin J.L., Coleman R.L., Obermair A. Minimally invasive versus abdominal radical hysterectomy for cervical cancer. N. Engl. J. Med. 2018;379:1895–1904. doi: 10.1056/NEJMoa1806395. [DOI] [PubMed] [Google Scholar]
- 12.Ferreira da Silva I., Koifman R.J. Cervical cancer treatment delays and associated factors in a cohort of women from a developing country. J Glob Oncol. 2019;5:1–11. doi: 10.1200/JGO.18.00199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ramey S.J., Asher D., Kwon D., Ahmed A.A., Wolfson A.H., Yechieli R., Portelance L. Delays in definitive cervical cancer treatment: an analysis of disparities and overall survival impact. Gynecol. Oncol. 2018;149:53–62. doi: 10.1016/j.ygyno.2017.12.010. [DOI] [PubMed] [Google Scholar]
- 14.Perri T., Issakov G., Ben-Baruch G., Felder S., Beiner M.E., Helpman L., Hogen L., Jakobson-Setton A., Korach J. Effect of treatment delay on survival in patients with cervical cancer: a historical cohort study. Int. J. Gynecol. Cancer. 2014;24:1326–1332. doi: 10.1097/IGC.0000000000000211. [DOI] [PubMed] [Google Scholar]
- 15.Shen S.C., Hung Y.C., Kung P.T., Yang W.H., Wang Y.H., Tsai W.C. Factors involved in the delay of treatment initiation for cervical cancer patients: a nationwide population-based study. Medicine (Baltimore) 2016;95 doi: 10.1097/MD.0000000000004568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nanthamongkolkul K., Hanprasertpong J. Longer waiting times for early stage cervical cancer patients undergoing radical hysterectomy are associated with diminished long-term overall survival. J. Gynecol. Oncol. 2015;26:262–269. doi: 10.3802/jgo.2015.26.4.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Umezu T., Shibata K., Kajiyama H., Yamamoto E., Mizuno M., Kikkawa F. Prognostic factors in stage IA-IIA cervical cancer patients treated surgically: does the waiting time to the operation affect survival? Arch. Gynecol. Obstet. 2012;285:493–497. doi: 10.1007/s00404-011-1966-y. [DOI] [PubMed] [Google Scholar]
- 18.E C., Dahrouge S., Samant R., Mirzaei A., Price J. Radical radiotherapy for cervix cancer: the effect of waiting time on outcome. Int. J. Radiat. Oncol. Biol. Phys. 2005;61:1071–1077. doi: 10.1016/j.ijrobp.2004.09.030. [DOI] [PubMed] [Google Scholar]
- 19.Girinsky T., Rey A., Roche B., Haie C., Gerbaulet A., Randrianarivello H., Chassagne D. Overall treatment time in advanced cervical carcinomas: a critical parameter in treatment outcome. Int. J. Radiat. Oncol. Biol. Phys. 1993;27:1051–1056. doi: 10.1016/0360-3016(93)90522-w. [DOI] [PubMed] [Google Scholar]
- 20.Hsieh G.L., Linesch S., Sajjad A., Macdonald A., Bonnen M., Anderson M.L., Ludwig M.S. Treatment compliance and outcomes for women with locoregionally advanced cervical cancer treated in a safety net health system. Int. J. Gynecol. Cancer. 2015;25:1669–1676. doi: 10.1097/IGC.0000000000000537. [DOI] [PubMed] [Google Scholar]
- 21.Jhawar S., Hathout L., Elshaikh M.A., Beriwal S., Small W., Mahmoud O. Adjuvant chemoradiation therapy for cervical cancer and effect of timing and duration on treatment outcome. Int. J. Radiat. Oncol. Biol. Phys. 2017;98:1132–1141. doi: 10.1016/j.ijrobp.2017.03.045. [DOI] [PubMed] [Google Scholar]
- 22.Matsuo K., Moeini A., Machida H., Fullerton M.E., Shabalova A., Brunette L.L., Roman L.D. Significance of venous thromboembolism in women with cervical cancer. Gynecol. Oncol. 2016;142:405–412. doi: 10.1016/j.ygyno.2016.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matsuo K., Purushotham S., Jiang B., Mandelbaum R.S., Takiuchi T., Liu Y., Roman L.D. Survival outcome prediction in cervical cancer: Cox models vs deep-learning model. Am J Obstet Gynecol. 2019;220:381 e1–381 e14. doi: 10.1016/j.ajog.2018.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matsuo K., Machida H., Mandelbaum R.S., Konishi I., Mikami M. Validation of the 2018 FIGO cervical cancer staging system. Gynecol. Oncol. 2019;152:87–93. doi: 10.1016/j.ygyno.2018.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matsuo K., Nusbaum D.J., Machida H., Huang Y., Khetan V., Matsuzaki S., Klar M., Grubbs B.H., Roman L.D., Wright J.D. Populational trends and outcomes of postoperative radiotherapy for high-risk early-stage cervical cancer with lymph node metastasis: concurrent chemo-radiotherapy versus radiotherapy alone. Am J Obstet Gynecol. 2020;222:484.e1–484.e15. doi: 10.1016/j.ajog.2019.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Austin P.C., Stuart E.A. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat. Med. 2015;34:3661–3679. doi: 10.1002/sim.6607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mandelbaum R., Ciccone M., Nusbaum D., Khoshchehreh M., Purswani H., Morocco E., Smith M., Matsuzaki S., Dancz C., Ozel B., Roman L., Paulson R., Matsuo K. Progestin therapy for obese women with complex atypical hyperplasia: levonorgestrel-releasing intrauterine device versus systemic therapy. Am J Obstet Gynecol. 2020 doi: 10.1016/j.ajog.2019.12.273. in-press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matsuo K., Matsuzaki S., Nusbaum D.J., Machida H., Nagase Y., Grubbs B.H., Roman L.D., Wright J.D., Harter P., Klar M. Malignant peritoneal cytology and decreased survival of women with stage I endometrioid endometrial cancer. Eur J Cancer. 2020;133:33–46. doi: 10.1016/j.ejca.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cohen J. 2nd ed. Erlbaum; Hillsdale, NJ: 1988. Statistical Power Analysis for the Behavioral Sciences. [Google Scholar]
- 30.von Elm E., Altman D.G., Egger M., Pocock S.J., Gotzsche P.C., Vandenbroucke J.P., Initiative S. Strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ. 2007;335:806–808. doi: 10.1136/bmj.39335.541782.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moher D., Liberati A., Tetzlaff J., Altman D.G., Group P Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maps & trends Johns Hopkins Universit of Medine Coronavirus resource center. https://coronavirus.jhu.edu/map.html>
- 33.Coronavirus Disease (COVID-19). Center for Disease Control and Prevention. 2019. https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html
- 34.United States Cancer Statistics: data visualizations. Center for Disease Control and Prevention. https://gis.cdc.gov/Cancer/USCS/DataViz.html>
- 35.Takushi M., Moromizato H., Sakumoto K., Kanazawa K. Management of invasive carcinoma of the uterine cervix associated with pregnancy: outcome of intentional delay in treatment. Gynecol. Oncol. 2002;87:185–189. doi: 10.1006/gyno.2002.6813. [DOI] [PubMed] [Google Scholar]
- 36.Bigelow C.A., Horowitz N.S., Goodman A., Growdon W.B., Del Carmen M., Kaimal A.J. Management and outcome of cervical cancer diagnosed in pregnancy. Am J Obstet Gynecol. 2017;216:276.e1–276.e6. doi: 10.1016/j.ajog.2016.10.034. [DOI] [PubMed] [Google Scholar]
- 37.Germann N., Haie-Meder C., Morice P., Lhomme C., Duvillard P., Hacene K., Gerbaulet A. Management and clinical outcomes of pregnant patients with invasive cervical cancer. Ann. Oncol. 2005;16:397–402. doi: 10.1093/annonc/mdi084. [DOI] [PubMed] [Google Scholar]
- 38.Gungorduk K., Sahbaz A., Ozdemir A., Gokcu M., Sancı M., Köse M.F. Management of cervical cancer during pregnancy. J. Obstet. Gynaecol. 2016;36:366–371. doi: 10.3109/01443615.2015.1065235. [DOI] [PubMed] [Google Scholar]
- 39.Hecking T., Abramian A., Domröse C., Engeln T., Thiesler T., Leutner C., Gembruch U., Keyver-Paik M.D., Kuhn W., Kübler K. Individual management of cervical cancer in pregnancy. Arch. Gynecol. Obstet. 2016;293:931–939. doi: 10.1007/s00404-015-3980-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Amant F., Halaska M.J., Fumagalli M., Dahl Steffensen K., Lok C., Van Calsteren K., Han S.N., Mir O., Fruscio R., Uzan C., Maxwell C., Dekrem J., Strauven G., Mhallem Gziri M., Kesic V., Berveiller P., van den Heuvel F., Ottevanger P.B., Vergote I., Lishner M., Morice P., Nulman I., Pregnancy EtfCi Gynecologic cancers in pregnancy: guidelines of a second international consensus meeting. Int. J. Gynecol. Cancer. 2014;24:394–403. doi: 10.1097/IGC.0000000000000062. [DOI] [PubMed] [Google Scholar]
- 41.Korenaga T.K., Tewari K.S. Gynecologic cancer in pregnancy. Gynecol Oncol. 2020 doi: 10.1016/j.ygyno.2020.03.015. in-press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pothuri B., Secord A.A., Armstrong D., Chan J., Hu W., Kesterson J., Liu J., Moore K., Fader A.N., Westin S., Naumann W. Anti-cancer therapy and clinical trial consideration for gynecologic oncology patients during the COVID-19 pandemic crisis. Gynecol Oncol. 2020 doi: 10.1016/j.ygyno.2020.04.694. in-press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Landoni F., Maneo A., Colombo A., Placa F., Milani R., Perego P., Favini G., Ferri L., Mangioni C. Randomised study of radical surgery versus radiotherapy for stage Ib-IIa cervical cancer. Lancet. 1997;350:535–540. doi: 10.1016/S0140-6736(97)02250-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material