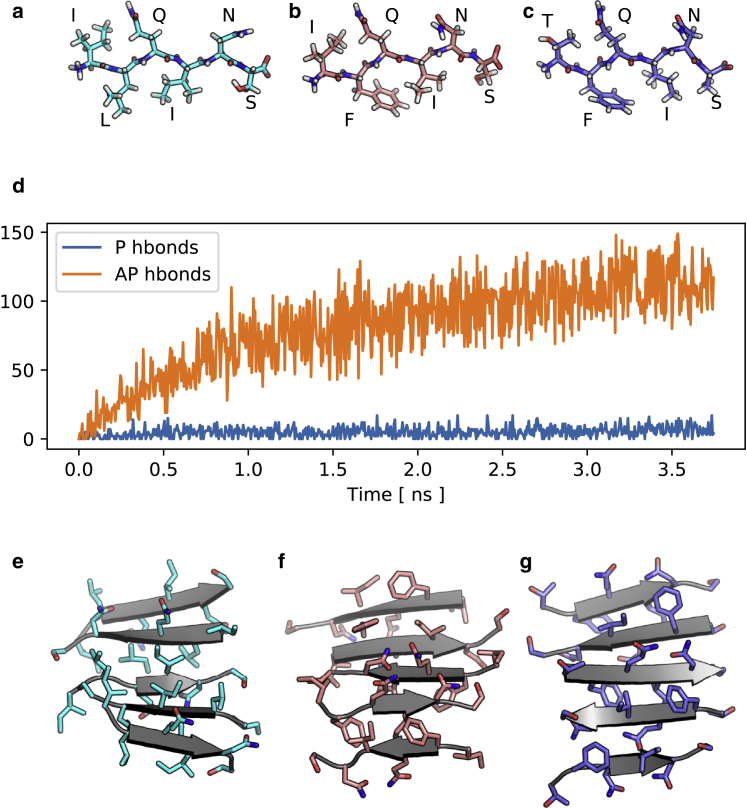
Figure 3.
Replica exchange. (a–c) Shown are the extended reference peptides used for HREMD simulations; these peptides have a pleated conformation compatible with the P structure. (d) AP sheets form very quickly in the accelerated simulation systems, preventing formation of the P sheet by absorbing free monomers (the example trace for ILQINS is shown). (e–g) Representative AP single β-sheets formed in these simulations are shown. Side-chain orientation in the AP sheets initially formed is not regular; the structures (e–g) are mixed between the intrasheet orderings of classes 5 and 6 and of 7 and 8. To see this figure in color, go online.