Figure 2. YqgP cleaves the high‐affinity magnesium transporter MgtE between its first and second transmembrane helices.
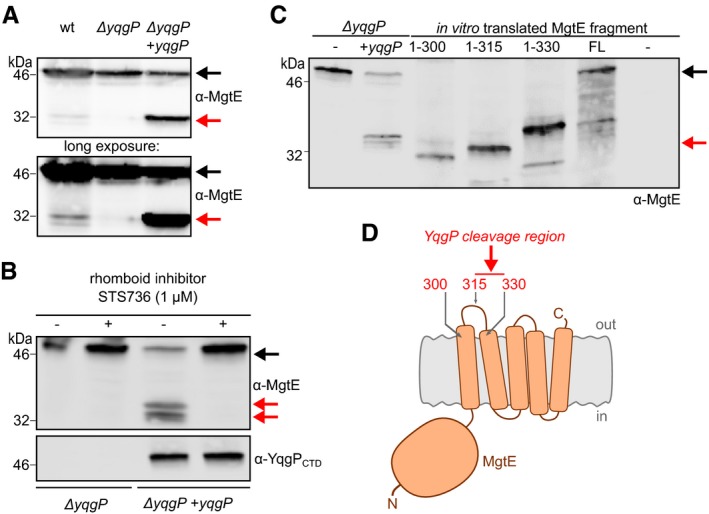
- Steady‐state cleavage profile of endogenous MgtE processed by endogenous and ectopically overexpressed YqgP from the inducible Phyperspank promoter in living Bacillus subtilis (BTM2 and BTM501, respectively, Table EV2), in minimal medium at low magnesium concentration (10 μM). Strain lacking YqgP (ΔyqgP, BTM78, Table EV2) was used as a control. Proteins were detected by immunoblotting with chemiluminescence detection.
- The cleavage is efficiently inhibited by 1 μM STS736, a specific peptidyl ketoamide rhomboid inhibitor (Ticha et al, 2017b).
- To map the cleavage site region within endogenous MgtE (second lane from the left, from strain BTM501), MgtE‐derived reference fragments encoding first 300, 315 and 330 amino acids, as well as full‐length MgtE (black arrow), were in vitro‐transcribed and in vitro‐translated. Mobility of the N‐terminal cleavage product (red arrow) of MgtE on SDS–PAGE was compared to the mobilities of the translated reference fragments.
- Diagrammatic display of the mapping shows that YqgP cleaves MgtE in a periplasmic region between transmembrane helices 1 and 2 (red arrow).
