Figure 8. Summary of the role of YqgP in MgtE proteostasis.
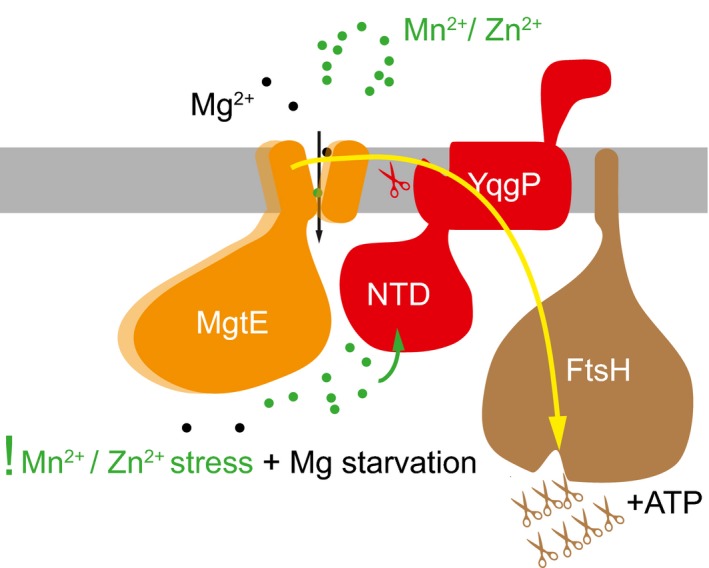
Under conditions of magnesium starvation, MgtE is upregulated and open, poised to transport Mg2+ inside the cell. If other divalent cations, such as Mn2+ or Zn2+, are present in the environment at relatively high concentrations, MgtE can transport them or be inhibited by them, which causes toxicity. Under these conditions, the N‐terminal cytosolic domain of YqgP binds Mn2+ or Zn2+, and activates YqgP for cleavage of MgtE between its transmembrane helices 1 and 2. YqgP also interacts with FtsH, and presents MgtE or its cleavage product(s) for processive proteolysis by FtsH. The cleavage of MgtE by YqgP is required for its full dislocation and degradation by FtsH. The presentation/adaptor function of YqgP is not dependent on its catalytic activity, but it requires its active site unoccupied. YqgP thus behaves also as a pseudoprotease which is common in the rhomboid superfamily. The connection of a rhomboid‐like protease/pseudoprotease (YqgP) with a processive degradative membrane‐bound protease/dislocase (FtsH) and its role in membrane protein quality control in Bacillus subtilis that we identify here represent a striking analogy to the eukaryotic role of rhomboid‐like pseudoproteases Derlins in ER‐associated degradation.
