Figure 1. Timecourse of ER stress responses and the role of PDI.
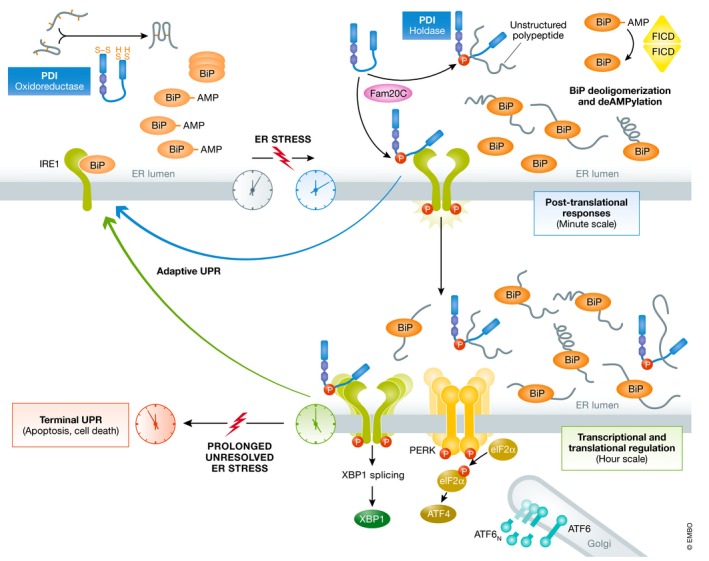
In non‐stress conditions, PDI (in blue) is acting as a pro‐folding enzyme, through the reduction, oxidation, and reshuffling of disulfide bonds. The UPR is kept inactivate e.g. by the stabilization of monomeric IRE1 (in green) through BiP binding (in orange); BiP itself is partially inactivated through oligomerization and AMPylation. Minutes after the begin of ER stress, Fam20C kinase (in pink) phosphorylates PDI at Ser357 promoting an open conformation which enhances PDIs holdase activity, thus enabling interaction with unfolded peptides and protection against aggregation in the ER lumen. In the same timescale, the pool of inactive BiP is activated by (i) deoligomerization, and (ii) deAMPylation carried out by FICD dimers. BiP displacement from IRE1 leads to its dimerization, trans‐autophosphorylation, and splicing of XBP1 transcripts. Phosphorylated PDI is able to bind IRE1, and this phosphorylation‐dependent interaction modulates the adaptive UPR. Prolonged, unsolvable ER stress leads to (hyper)activation of the different UPR branches and will ultimately induce apoptosis.
