Abstract
Aims/Introduction
A retrospective study was carried out to investigate the clinical characteristics and associated factors for invasive fungal disease in patients with type 2 diabetes mellitus.
Materials and Methods
Demographic and clinical data were recorded. Associated factors were analyzed by logistic regression analysis.
Results
Invasive fungal disease was diagnosed in 120 patients with type 2 diabetes mellitus (prevalence, 0.4%). Yeast infection (56/120, 46.7%), including candidiasis (31/56, 55.4%) and cryptococcosis (25/56, 44.6%), was the most common. The urinary tract was mainly involved in candidiasis (12/31, 38.7%). More than half of the cryptococcosis (16/25, 64.0%) presented as pneumonia. Mold infection accounted for 40.8% of the cases, and predominantly involved the lung (34/49, 69.4%). A total of 15 (12.5%) patients had mixed fungal infection. Candida albicans (24/111, 21.6%), Cryptococcus neoformans (19/111, 17.1%) and Aspergillus fumigatus (14/111, 12.6%) were the leading agents. Co‐infection occurred in 58 (48.3%) patients, mainly presenting as pneumonia caused by Gram‐negative bacteria. The inpatient mortality rate of invasive fungal disease was 23.3% (28/120). Glycated hemoglobin levels were higher in non‐survivors than survivors (8.8 ± 2.5 vs 7.7 ± 2.1%, P = 0.02). Anemia (adjusted odds ratio, 3.50, 95% confidence interval 1.95–6.27, P < 0.001), hypoalbuminemia (adjusted odds ratio, 5.42, 95% confidence interval 3.14–9.36, P < 0.001) and elevated serum creatinine (adjusted odds ratio, 2.08, 95% confidence interval 1.07–4.04, P = 0.03) were associated with invasive fungal disease in type 2 diabetes mellitus patients.
Conclusions
Invasive fungal disease is a life‐threatening complication in type 2 diabetes mellitus patients. C. a albicans, C. neoformans, and A. fumigatus are the leading agents. Prolonged hyperglycemia results in unfavorable outcomes. Correction of anemia and hypoalbuminemia might improve prognosis.
Keywords: Diabetes mellitus, Diabetic nephropathy, Invasive fungal disease
We investigated the characteristics and associated factors of invasive fungal disease in patients with type 2 diabetes mellitus from Southern China for the first time. We found that Candida albicans, Cryptococcus neoformans and Aspergillus fumigatus were the leading agents. Prolonged hyperglycemia results in unfavorable outcomes. Correction of anemia and hypoalbuminemia might improve prognosis.

Introduction
Diabetes mellitus is a group of metabolic disorders characterized by high blood glucose levels over a prolonged period. Patients with diabetes are susceptible to infection and usually require more hospitalization, compared with the general population1, 2. With the productive management of diabetic complications, the proportional mortality due to vasculopathy has declined, offset by non‐vascular causes, including infection, during the past three decades3. In developing countries, infection is one of the three leading causes of deaths in patients with diabetes, and increases the excess risk to fourfold4.
Invasive fungal disease (IFD) is a life‐threatening infection with high mortality. The attributable mortality of invasive candidiasis is considered to range from 10 to 15%, and that of invasive aspergillosis is 42–64%5 in critically ill patients. IFD in diabetes patients presents different characteristics. Diabetes patients are vulnerable to fungal infection. The risk of mycoses increases 1.38‐fold in patients with diabetes6, and diabetes is widely recognized as a risk factor for invasive pulmonary aspergillosis5. Uncontrolled hyperglycemia contributes to a poor prognosis of type 2 diabetes patients with cryptococcosis7. Diabetes is a potential risk factor for IFD caused by unusual fungi, such as Histoplasma capsulatum 8. Fungi in patients with diabetes present increasing drug resistance. Biofilm is a major physical barrier to reducing the absorption of antifungals, leading to antifungal tolerance9, 10, 11. Candida spp. isolated from the oral cavity from type 2 diabetes patients are more likely to form biofilm than those in non‐diabetic individuals12, 13.
China has the largest burden of diabetes 14. China is also an epidemic region for IFD15. To determine the epidemiology and clinical features of IFD in Chinese diabetes patients, early recognition and treatment is required. Herein, we carried out a retrospective study, aiming to investigate the clinical characteristics and associated factors of IFD in adult patients with type 2 diabetes from Southern China.
Methods
Study design
A retrospective study was carried out with inpatients aged ≥14 years from the First Affiliated Hospital of Sun Yat‐Sen University, Guangzhou, China, from 1 January 2013 to 31 December 2018. The International Classification of Diseases 10th revision coding of discharged diagnoses was used to identify patients with type 2 diabetes and IFD (Appendix 1). One episode of IFD from each patient was collected. A total of 30,984 patients with type 2 diabetes were screened, and 122 of them had IFD. The 122 records were re‐evaluated by two physicians (Minxi Lao and Yingying Gong) using the diagnostic criteria shown in Appendix 2. One patient was excluded, because IFD was diagnosed before type 2 diabetes diagnosis. One patient was excluded for incomplete data. Finally, 120 patients with type 2 diabetes and IFD were included. The enrolling patients were subcategorized by comorbidities. The average age and duration of type 2 diabetes were calculated within groups. Patients with type 2 diabetes, but no infection during the same period of hospitalization, were chosen and classified by comorbidities. In each subgroup, controls with age between the age of cases ± 5 years and disease duration between duration of cases ± 1 year were selected. Then, we re‐selected the control patients randomly from age and disease duration‐matching subgroups with different comorbidities. The total number of controls were set as twice the number of the cases (Figure 1). The ethics committee of the First Affiliated Hospital of Sun Yat‐sen University approved the research (approval number: 2019422), waiving written informed consent for deidentified patient data. This work was carried out according to the provisions of the Declaration of Helsinki16.
Figure 1.
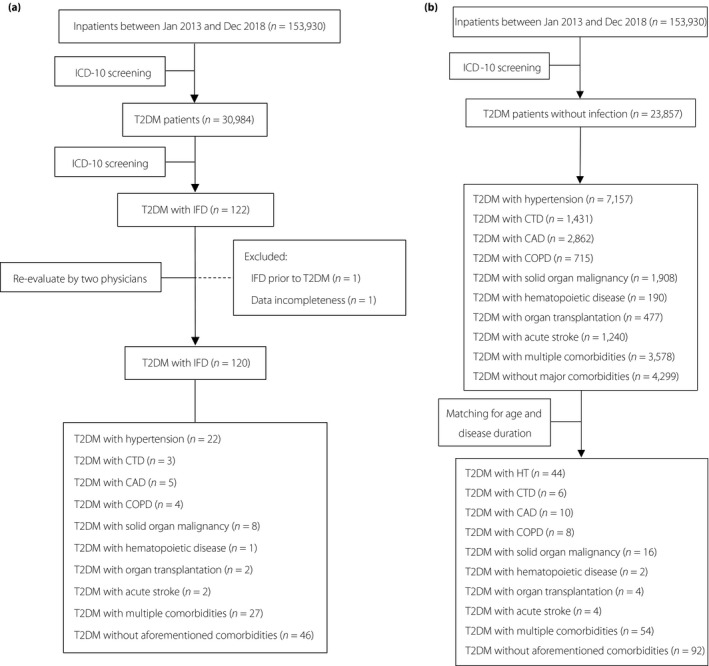
Screening algorithm. (a) The screening algorithm for patients with type 2 diabetes (T2DM) and invasive fungal disease (IFD). (b) The screening algorithm for control cases. CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; CTD, connective tissue disease; HT, hypertension; ICD, International Classification of Diseases.
Definition of cases
Diabetes mellitus was defined according to Chinese guidelines for type 2 diabetes 17. Diabetic nephropathy was diagnosed based on the measurement of abnormal levels of urinary albumin (≥30 mg/24 h) in a diabetes patient coupled with exclusion of other causes of albuminuria18.
The diagnostic criteria for IFD are shown in Appendix 2. IFD was designated as proven, probable or possible according to the European Organisation for Research and Treatment of Cancer/Mycoses Study Group (EORTC/MSG) Guidelines19, 20, and Chinese expert consensus on diagnosis and treatment of candidiasis21. Nosocomial infection was defined as infection acquired in a hospital or infection that originates in a hospital, but shows symptoms after discharge. Accordingly, infection initiating 48 h after admission was considered nosocomial infection. Disseminated IFD was defined in the case of fungemia or IFD that involved two or more non‐consecutive organs. Fungal infection only involving skin, genitalia and the oral cavity was considered as a superficial fungal infection, and was excluded. No patient with type 2 diabetes and histoplasmosis, blastomycosis, coccidioidomycosis, paracoccidioidomycosis, sporotrichosis or infection due to Talaromyces marneffei was found in our database.
Co‐infection with bacteria was considered as proven or probable according to etiology evidence. Proven infection was confirmed if pathogens were identified by microscopy or culture. Probable infection was diagnosed on the basis of clinical symptoms, radiographic imaging, indirect laboratory data and treatment response to antibiotics. Superficial fungi infection was defined as fungi infection that occurred in the oral cavity, skin/mucosa or genitalia with culture evidence. Acute viral infection was diagnosed on the basis of clinical and radiographic manifestations, positivity for antiviral immunoglobulin (Ig) M antibodies and subsequent specific IgG antibodies, and replication of the virus was confirmed by molecular methods, such as polymerase chain reaction.
Demographical and clinical data
Demographic and clinical data were collected from the medical records. Clinical characteristics included diabetic complications, potential risk factors, and invasive procedures within the 3 months before IFD. Accumulated dose of glucocorticoid (GC) during the 3 months before IFD was summed and converted to equivalent prednisolone using the following equation: 1 mg of prednisone = 0.8 mg of methylprednisolone = 0.15 mg of dexamethasone. Clinical characteristics of IFD included symptoms and signs, sites of infection, and antifungi treatment. Laboratory data included routine blood tests, blood glucose level and glycated hemoglobin (HbA1c). Hypoalbuminemia was defined as serum albumin level <3.5 g/dL. β‐D‐glucan detection and galactomannan antigen test were carried out according to the manufacturer’s type 2 diabetes instructions. Cryptococcal capsule polyglycan antigens were detected by latex agglutination test. Microbial culture and biopsy findings were recorded if available.
Statistical analysis
Quantitative variables are expressed as the mean ± standard deviation or median (interquartile range), and qualitative variables as absolute numbers and percentages. Proportions were compared using Fisher’s exact test or Pearson’s χ2‐test. According to the study design, controls were not strictly matched to cases by 2:1. Therefore, between‐group comparison was evaluated using Student’s t‐test for continuous variables with normal distribution or Mann–Whitney U‐test for continuous variables with non‐normal distribution. The odds ratio (OR) and corresponding 95% confidence interval (CI) of clinically significant variables with a P < 0.1 in between‐group comparison was adjusted by multivariate logistic regression analysis to identify the associated factors of infection. The forward procedure was applied in the multivariate logistic regression analysis. The two‐sided level of significance was set at P < 0.05. All statistical analysis was carried out with the SPSS 19 statistical package (IBM Corporation, Armonk, NY, USA).
Results
Demographics
A total of 120 patients (73 men, 47 women) with type 2 diabetes were included. The mean (standard deviation) age was 60.5 ± 11.7 years (range 15–88 years). The median (interquartile range) duration of type 2 diabetes at IFD onset was 54 months (5.5–120 months). Diabetic nephropathy was diagnosed in 36 (30.0%) patients. No patient was infected with human immunodeficiency virus. The prevalence of IFD in type 2 diabetes inpatients was 0.4% (120/30,984). By contrast, the prevalence of IFD in non‐diabetes inpatients from the same institution was 0.2% (249/119,255). Proven cases accounted for 51.7% (62/120), probable cases for 30.8% (37/120) and possible cases for 17.5% (21/120). Nosocomial infection accounted for 33.3% (40/120). A total fo 81 (67.5%) patients were receiving treatment for type 2 diabetes before IFD onset. Insulin alone was prescribed to 34 (42.0%) patients, oral antidiabetic drugs to 40 (49.4%), and combined therapy of insulin and oral antidiabetic drugs to seven (8.6%) patients. The average (stabndard deviation) HbA1c level was 8.0 ± 2.2%.
Characteristics of yeast infection
A total of 56 (46.7%) patients had yeast infection, among which 31 (55.4%) developed candidiasis and 25 (44.6%) developed cryptococcosis. The characteristics of candidiasis are shown in Table 1. The urinary tract was the most commonly involved (12/31, 38.7%), followed by the abdominal cavity (10/31, 32.3%; Figure 2a). All the patients developing intraabdominal candidiasis (IAC) received gastrointestinal operation before IFD. The positive rate of β‐D‐glucan detection in candidal infection was 47.4% (9/19).
Table 1.
Characteristics of type 2 diabetes patients with invasive fungal disease
| Yeast infection (n = 56) | Mold infection (n = 49) | Mixed fungal infection (n = 15) | ||
|---|---|---|---|---|
| Candidiasis (n = 31) | Cryptococcosis (n = 25) | |||
| Demographic characteristics | ||||
| Sex (male:female) | 20:11 | 14:11 | 30:19 | 9:6 |
| Age, years (mean ± SD) | 60.5 ± 15.3 | 61.0 ± 7.5 | 61.6 ± 10.5 | 56.2 ± 12.7 |
| BMI, kg/m2 (mean ± SD) | 22.4 ± 3.2 | 22.3 ± 2.9 | 20.6 ± 3.1 | 22.8 ± 3.6 |
| Smoker, n (%) | 13 (41.9) | 3 (12.0) | 20 (40.8) | 4 (26.7) |
| Comorbidities | ||||
| Hypertension, n (%) | 13 (41.9) | 13 (52.0) | 15 (30.6) | 5 (33.3) |
| CTD, n (%) | 1 (3.2) | 4 (16.0) | 4 (8.2) | 3 (20.0) |
| CAD, n (%) | 5 (16.1) | 4 (16.0) | 4 (8.2) | 2 (13.3) |
| COPD, n (%) | 1 (3.2) | 0 (0) | 5 (10.2) | 0 (0) |
| Solid organ malignancy, n (%) | 6 (19.4) | 2 (8.0) | 3 (6.1) | 2 (13.3) |
| Hematopoietic disease, n (%) | 0 (0) | 0 (0) | 2 (4.1) | 0 (0) |
| Organ transplantation, n (%) | 2 (6.5) | 0 (0) | 2 (4.1) | 2 (13.3) |
| Acute stroke, n (%) | 0 (0) | 1 (4.0) | 2 (4.1) | 0 (0) |
| Characteristics of T2DM | ||||
| Median duration of T2DM, months (IQR) | 84 (24‐120) | 48 (3‐120) | 48 (6‐120) | 24 (2.5‐84) |
| Diabetic ketoacidosis, n (%) | 0 (0) | 0 (0) | 1 (2.0) | 2 (13.3) |
| Diabetic nephropathy, n (%) | 16 (51.6) | 4 (16.0) | 10 (20.4) | 6 (40.0) |
| HbA1c, % (mean ± SD) | 7.4 ± 2.0 | 7.7 ± 1.9 | 8.5 ± 2.3 | 7.7 ± 2.7 |
| Risk factors | ||||
| ICU admission, n (%) | 4 (12.9) | 2 (8.0) | 7 (14.3) | 7 (46.7) |
| Use of broad‐spectrum antibiotics, n (%) | 21 (67.7) | 3 (12.0) | 18 (36.7) | 11 (73.3) |
| Use of GC, n (%) | 1 (3.2) | 5 (20.0) | 3 (6.1) | 4 (26.7) |
| Immunosuppressants/chemotherapy, n (%) | 3 (9.7) | 2 (8.0) | 4 (8.2) | 5 (33.3) |
| Operation, n (%) | 11 (35.5) | 0 (0) | 3 (6.1) | 6 (40.0) |
| Dialysis‐dependent, n (%) | 3 (9.7) | 0 (0) | 4 (8.2) | 0 (0) |
| Indwelling urinary catheter, n (%) | 6 (19.4) | 0 (0) | 9 (18.4) | 7 (46.7) |
| Central venous catheter, n (%) | 11 (35.5) | 2 (8.0) | 8 (16.3) | 9 (60.0) |
| Total parenteral nutrition, n (%) | 5 (16.1) | 0 (0) | 0 (0) | 2 (13.3) |
| Inpatient mortality, n (%) | 7 (22.6) | 3 (12.0) | 10 (20.4) | 8 (53.3) |
BMI, body mass index; CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; CTD, connective tissue disease; GC, glucocorticoid; ICU, intensive care unit; IFD, invasive fungal disease; IQR, interquartile range; SD, standard deviation; T2DM, type 2 diabetes mellitus.
Figure 2.
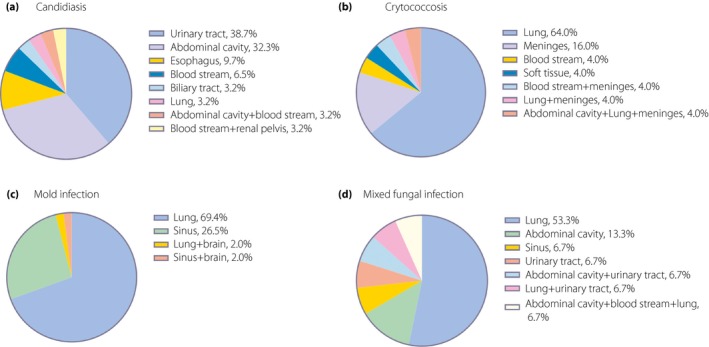
Anatomic sites of invasive fungal disease (IFD) in patients with type 2 diabetes. (a) Infected sites of candidiasis. (b) Infected sites of cryptococcosis. (c) Infected sites of mold infection. (d) Infected sites of mixed fungal infection.
Cryptococcosis was diagnosed in 25 patients (Table 1). Four (16.0%) patients were complicated with connective tissue disease (CTD), and one fifth (5/25) were taking GC before IFD. More than half of cryptococcosis (16/25, 64.0%) developed in the lung, and 16% (4/25) occurred in meninges (Figure 2b). Serum cryptococcal capsule polyglycan antigen was positive in 16 out of 20 patients (80.0%).
Characteristics of mold infection
A total of 49 (40.8%) patients with type 2 diabetes developed mold infection (Table 1). The lung (34/49, 69.4%) was the most frequently involved, followed by sinus (13/49, 26.5%; Figure 2c). Positivity of serum galactomannan antigen test and bronchoalveolar lavage fluid galactomannan antigen test was 41.2% (14/34) and 73.3% (11/15), respectively.
Characteristics of mixed fungal infection
Mixed fungal infection developed in 15 (12.5%) patients with type 2 diabetes (Table 1). The proportions of patients having CTD (3/15, 20%) or organ transplantation (2/15, 13.3%) were the highest in this group. Similarly, the largest number of patients who received GC (4/15, 26.7%) or immunosuppressants (5/15, 33.3%) was observed. The lung (8/15, 53.3%) was the most frequently involved, followed by the abdominal cavity (2/15, 13.3%; Figure 2d).
Characteristics of IFD in type 2 diabetes patients under specific conditions
The characteristics of community‐acquired and nosocomial IFD are shown in Table 2. Patients with nosocomial IFD were older (aged 63.8 ± 12.9 vs 58.9 ± 10.7 years, P = 0.03), having longer disease duration (82 vs 36 months, P = 0.003) and higher proportion of diabetic nephropathy (47.5% vs 21.3%, P = 0.003). Candidiasis is the major infectious pattern in nosocomial IFD (55.0% vs 11.3%, P < 0.001).
Table 2.
Comparison between community‐acquired and nosocomial invasive fungal disease in patients with type 2 diabetes mellitus
| Community‐acquired (n = 80) | Nosocomial (n = 40) | P‐value | |
|---|---|---|---|
| Demographic characteristics | |||
| Sex (male : female) | 49:31 | 24:16 | 0.89 |
| Age, years (mean ± SD) | 58.9 ± 10.7 | 63.8 ± 12.9 | 0.03* |
| BMI, kg/m2 (mean ± SD) | 21.7 ± 3.5 | 21.7 ± 2.7 | 0.94 |
| Smoker, n (%) | 23 (28.8) | 17 (42.5) | 0.10 |
| Comorbidities | |||
| Hypertension, n (%) | 28 (35.0) | 18 (45.0) | 0.29 |
| CTD, n (%) | 12 (15.0) | 0 (0) | 0.01* |
| CAD, n (%) | 6 (7.5) | 9 (22.5) | 0.02* |
| COPD, n (%) | 4 (5.0) | 2 (5.0) | 1.00 |
| Solid organ malignancy, n (%) | 7 (8.8) | 6 (15.0) | 0.001* |
| Hematopoietic disease, n (%) | 2 (2.5) | 0 (0) | 0.31 |
| Organ transplantation, n (%) | 2 (2.5) | 4 (10.0) | 0.08 |
| Acute stroke, n (%) | 2 (2.5) | 1 (2.5) | 1.00 |
| Characteristics of T2DM | |||
| Median duration of T2DM, months (IQR) | 36 (2–120) | 82 (36–180) | 0.003* |
| Diabetic ketoacidosis, n (%) | 2 (2.5) | 1 (2.5) | 1.00 |
| Diabetic nephropathy, n (%) | 17 (21.3) | 19 (47.5) | 0.003* |
| HbA1c, % (mean ± SD) | 8.0 ± 2.2 | 7.8 ± 2.3 | 0.56 |
| Risk factors | |||
| Use of GC, n (%) | 11 (13.8) | 2 (5.0) | 0.15 |
| Median accumulated dose of PSL, mg (IQR) | 1,417.5 (487.5–2,952.5) | 457 (453.5–460.5) | 0.36 |
| Immunosuppressants/chemotherapy, n (%) | 10 (12.5) | 4 (10.0) | 0.69 |
| Operation, n (%) | 1 (1.3) | 19 (47.5) | <0.001* |
| Dialysis‐dependent, n (%) | 1 (1.3) | 6 (15.0) | 0.002* |
| Indwelling urinary catheter, n (%) | 6 (7.5) | 16 (40.0) | <0.001* |
| Central venous catheter, n (%) | 10 (12.5) | 20 (50.0) | <0.001* |
| Total parenteral nutrition, n (%) | 0 (0) | 7 (17.5) | 0.001* |
| Infective sites | |||
| Lung, n (%) | 48 (60.0) | 11 (27.5) | 0.001* |
| Sinus, n (%) | 13 (16.3) | 1 (2.5) | 0.03* |
| CNS, n (%) | 4 (5.0) | 0 (0) | 0.15 |
| Abdominal cavity, n (%) | 3 (3.8) | 9 (22.5) | 0.001* |
| Esophagus, n (%) | 2 (2.5) | 1 (2.5) | 1.00 |
| Urinary tract, n (%) | 1 (1.3) | 12 (30.0) | <0.001* |
| Soft tissue, n (%) | 1 (1.3) | 0 (0) | 0.21 |
| Biliary tract, n (%) | 0 (0) | 1 (2.5) | 0.01* |
| Disseminated, n (%) | 8 (10.0) | 5 (12.5) | 0.81 |
| Infective patterns | |||
| Candidiasis, n (%) | 9 (11.3) | 22 (55.0) | <0.001* |
| Cryptococcosis, n (%) | 25 (31.3) | 0 (0) | 0.02* |
| Mold infection, n (%) | 37 (46.3) | 12 (30.0) | 0.13 |
| Mixed fungal infection, n (%) | 9 (11.3) | 6 (15.0) | 0.63 |
| Inpatient mortality, n (%) | 12 (15.0) | 16 (40.0) | <0.001* |
P < 0.05. BMI, body mass index; CAD, coronary artery disease; CNS, central nervous system; COPD, chronic obstructive pulmonary disease; CTD, connective tissue disease; GC, glucocorticoid; IFD, invasive fungal disease; IQR, interquartile range; PSL, prednisolone; SD, standard deviation; T2DM, type 2 diabetes mellitus.
Seven type 2 diabetes patients received chemotherapy before IFD onset. Three (42.9%) of them developed mold infection, two (28.6%) developed candidiasis and two (28.6%) developed mixed fungal infection. Two type 2 diabetes patients were complicated with hematopoietic disease, including one with acute lymphocytic leukemia and one with myelodysplastic syndrome. These two patients developed pulmonary aspergillosis and sinus mucormycosis, respectively. A total of 20 cases of IFD occurred postoperation. There was a trend toward candidiasis (11/20, 55.0%), followed by mixed fungal infection (6/20, 30.0%). The abdominal cavity was the predominant infective site (8/20, 40.0%). A total of 30 type 2 diabetes patients received central venous catheterization, and three (10.0%) of them developed fungemia. A total of 22 type 2 diabetes patients received indwelling urinary catheterization, and four (18.2%) of them developed a fungal urinary tract infection. IFD secondary to bacterial infection occurred in 31 (25.8%) patients. Aspergillosis (12/31, 38.7%) and candidiasis (11/31, 35.5%) were comparable. The lung (12/12, 100%) and urinary tract (6/11, 54.5%) were commonly affected.
Distribution of causative fungi
Causative fungi were isolated from sputum (n = 20), ascitic fluid (n = 16), urine (n = 16), bronchoalveolar lavage fluid (n = 10), blood (n = 6), cerebrospinal fluid (n = 5), esophageal brushing sample (n = 3), bile (n = 1), drain from a brain hematoma (n = 1) and drain from the renal pelvis (n = 1). Fungi were found in biopsied tissue or operative specimens from 34 patients. A total of 111 strains of fungi, including 64 (57.7%) strains of yeasts and 47 (42.3%) strains of mold, were identified (Table 3). Candida albicans (24/111, 21.6%) was the most common agent in yeast infection, followed by Cryptococcus neoformans (19/111, 17.1%). Aspergillus fumigatus was the major causative mold, accounting for 12.6% (14/111) of the cases. Data of antifungal susceptibility tests were available in 17 strains of C. albicans, eight strains of C. glabrata, seven strains of C. tropicalis and four strains of C. parapsilosis. The resistance rates of C. albicans against itraconazole, flucytosine and fluconazole were 11.8% (2/17), 5.9% (1/17) and 5.9% (1/17), respectively. Three (37.5%) strains of C. glabrata were resistant to itraconazole. The resistance rates of C. tropicalis against fluconazole, voriconazole and itraconazole were 28.6% (2/7), 14.3% (1/7) and 14.3% (1/7), respectively. Of note, one strain of C. tropicalis was resistant against fluconazole, voriconazole and itraconazole simultaneously.
Table 3.
Distribution of isolated fungi
| Isolated fungi | n (%) |
|---|---|
| Total | 111 |
| Yeast | 64 (57.7) |
| Candida albicans | 24 (21.6) |
| Candida glabrata | 9 (8.1) |
| Candida tropicalis | 8 (7.2) |
| Candida parapsilosis | 4 (3.6) |
| Candida krusei | 0 (0) |
| Cryptococcus neoformans | 19 (17.1) |
| Mold | 47 (42.3) |
| Aspergillus fumigatus | 14 (12.6) |
| Aspergillus flavus | 6 (5.4) |
| Aspergillus niger | 2 (1.8) |
| Aspergillus versicolor | 1 (0.9) |
| Aspergillus unclassified | 14 (12.6) |
| Mucor spp. | 8 (7.2) |
| Rhizopus spp. | 1 (0.9) |
| Fusarium spp. | 1 (0.9) |
Co‐infection in diabetes patients with IFD
Co‐infection with other agents was diagnosed in 58 (48.3%) patients. The lung was frequently involved (30/58, 51.7%). Most of the patients (54/58, 93.1%) were co‐infected with bacteria. Among 92 strains of bacteria isolated from 43 patients, 67 (72.8%) strains were Gram‐negative bacteria, and 25 (27.2%) strains were Gram‐positive bacteria. One (1/58, 1.7%) patient was co‐infected with active tuberculosis. Viral infection included pneumonia caused by influenza virus (n = 1), pneumonia caused by cytomegalovirus (n = 1) and viral meningitis (n = 1).
Treatment and outcomes of IFD
Antifungal treatment was documented in 108 (90.0%) patients. A total of 90 (83.3%) patients received antifungal medications, 12 (11.1%) patients underwent operation alone, and six (5.6%) patients were treated with operation and subsequent antifungal medications. The median follow‐up period was 0.9 months (interquartile range0.5–2.7 months). A majority of patients (92/108, 85.2%) showed disease improvement. The inpatient mortality rate was 23.3% (28/120). Septic shock was the primary cause (22/28, 78.6%), followed by acute stroke (2/28, 7.1%), heart failure (2/28, 7.1%), pleural hemorrhage (1/28, 3.6%) and asphyxia (1/28, 3.6%). The mortality was the highest in patients with mixed fungal infection (8/15, 53.3%), followed by mold infection (10/49, 20.4%) and yeast infection (10/56, 17.9%).
A comparison between survivors and non‐survivors was carried out (Table 4). Patients with poor prognosis were more likely to have diabetic nephropathy (50.0% vs 23.9%, P = 0.01), mixed fungal infection (28.6% vs 7.6%, P = 0.01), disseminated IFD (25.0% vs 6.5%, P = 0.01) and co‐infection (92.9% vs 34.8%, P < 0.001). Lymphopenia was more predominant in deceased patients (57.1% vs 21.7%, P < 0.001). HbA1c level was higher in non‐survivors (8.8 ± 2.5 vs 7.7 ± 2.1%, P = 0.02).
Table 4.
Comparison between survivors and non‐survivors with invasive fungal disease
| Survivors (n = 92) | Non‐survivors (n = 28) | P‐value | |
|---|---|---|---|
| Demographic characteristics | |||
| Sex (male : female) | 54:38 | 19:9 | 0.38 |
| Age, years (mean ± SD) | 60.3 ± 11.1 | 61.0 ± 13.6 | 0.79 |
| BMI, kg/m2 (mean ± SD) | 21.6 ± 3.2 | 22.1 ± 3.2 | 0.49 |
| Smoker, n (%) | 27 (29.3) | 13 (46.4) | 0.09 |
| Risk factors | |||
| ICU admission, n (%) | 1 (1.1) | 19 (67.9) | <0.001* |
| Use of broad‐spectrum antibiotics, n (%) | 30 (32.6) | 23 (82.1) | <0.001* |
| Use of GC, n (%) | 10 (10.9) | 3 (10.7) | 0.98 |
| Median accumulated dose of PSL, mg (IQR) | 875 (453.5–2,461.3) | 1,740 (1,095–2,385) | 0.56 |
| Immunosuppressants/chemotherapy, n (%) | 9 (9.8) | 5 (17.9) | 0.24 |
| Operation, n (%) | 8 (8.7) | 12 (42.9) | <0.001* |
| Dialysis‐dependent, n (%) | 6 (6.5) | 1 (3.6) | 0.56 |
| Indwelling urinary catheter, n (%) | 4 (4.3) | 18 (64.3) | <0.001* |
| Central venous catheter, n (%) | 7 (7.6) | 23 (82.1) | <0.001* |
| Total parenteral nutrition, n (%) | 5 (5.4) | 2 (7.1) | 0.74 |
| Characteristics of T2DM | |||
| Median duration of T2DM, months (IQR) | 48 (3.8–120) | 60 (12–168) | 0.13 |
| Diabetic ketoacidosis, n (%) | 0 (0) | 3 (10.7) | 0.001* |
| Diabetic nephropathy, n (%) | 22 (23.9) | 14 (50.0) | 0.01* |
| Characteristics of IFD | |||
| Agents | |||
| Yeast, n (%) | 46 (50.0) | 10 (35.7) | 0.07 |
| Mold, n (%) | 39 (42.4) | 10 (35.7) | 0.91 |
| Mixed fungi, n (%) | 7 (7.6) | 8 (28.6) | 0.01* |
| Disseminated IFD, n (%) | 6 (6.5) | 7 (25.0) | 0.01* |
| Co‐infection, n (%) | 32 (34.8) | 26 (92.9) | <0.001* |
| Laboratory data | |||
| Leukopenia, n (%) | 3 (3.3) | 2 (7.1) | 0.56 |
| Lymphopenia, n (%) | 20 (21.7) | 16 (57.1) | <0.001* |
| Anemia, n (%) | 48 (52.2) | 20 (71.4) | 0.07 |
| Hypoalbuminemia, n (%) | 54 (58.7) | 21 (75.0) | 0.12 |
| Elevated serum creatinine, n (%) | 29 (31.5) | 15 (53.6) | 0.03* |
| HbA1c, % (mean ± SD) | 7.7 ± 2.1 | 8.8 ± 2.5 | 0.02* |
P < 0.05. BMI, body mass index; GC, glucocorticoid; ICU, intensive care unit; IFD, invasive fungal disease; IQR, interquartile range; PSL, prednisolone; SD, standard deviation; T2DM, type 2 diabetes mellitus.
Comparison between type 2 diabetes patients with and without IFD
A comparison between diabetes patients with and without IFD is shown in Table 5. Diabetic nephropathy (30.0% vs 15.8%, P = 0.002) was more prevalent in the IFD group. Patients with IFD were more likely to have lymphopenia (30.0% vs 8.3%, P < 0.001), anemia (56.7% vs 15.0%, P < 0.001) and hypoalbuminemia (62.5% vs 16.3%, P < 0.001). Serum creatinine levels elevated remarkably in diabetes patients with IFD (36.7% vs 10.8%, P < 0.001).
Table 5.
Comparison between type 2 diabetes patients with and without invasive fungal disease
| Characteristics | Case (n = 120) | Control (n = 240) | P‐value |
|---|---|---|---|
| Demographic characteristics | |||
| Sex (male : female) | 73:47 | 150:90 | 0.76 |
| Age, year (mean ± SD) | 60.5 ± 11.7 | 61.1 ± 9.3 | 0.62 |
| BMI, kg/m2 (mean ± SD) | 21.7 ± 3.2 | 23.0 ± 3.1 | <0.001* |
| Smokers, n (%) | 40 (33.3) | 73 (30.4) | 0.57 |
| Risk factors | |||
| Use of GC, n (%) | 13 (10.8) | 21 (8.8) | 0.52 |
| Median accumulated dose of PSL, mg (IQR) | 875.0 (450.0–2,797.5) | 900 (450.0–1,350.0) | 0.27 |
| Immunosuppressants/chemotherapy, n (%) | 14 (11.7) | 23 (9.6) | 0.54 |
| Dialysis‐dependent, n (%) | 7 (5.8) | 5 (2.7) | 0.16 |
| Comorbidities | |||
| Hypertension, n (%) | 46 (38.3) | 105 (43.8) | 0.33 |
| CTD, n (%) | 12 (10.0) | 24 (10.0) | 1.00 |
| CAD, n (%) | 15 (12.5) | 41 (17.1) | 0.26 |
| COPD, n (%) | 6 (5.0) | 16 (6.7) | 0.53 |
| Solid organ malignancy, n (%) | 13 (10.8) | 33 (13.8) | 0.43 |
| Hematopoietic disease, n (%) | 2 (1.7) | 4 (1.7) | 1.00 |
| Organ transplantation, n (%) | 6 (5.0) | 12 (5.0) | 1.00 |
| Stroke, n (%) | 3 (2.5) | 16 (6.7) | 0.10 |
| Characteristics of T2DM | |||
| Median duration of T2DM, months (IQR) | 54 (5.5–120) | 60 (15.5–120) | 0.55 |
| Diabetic ketoacidosis, n (%) | 3 (2.5) | 2 (0.9) | 0.24 |
| Diabetic nephropathy, n (%) | 36 (30.0) | 38 (15.8) | 0.002* |
| Laboratory data | |||
| Leukopenia, n (%) | 5 (4.2) | 15 (6.3) | 0.42 |
| Lymphopenia, n (%) | 36 (30.0) | 20 (8.3) | <0.001* |
| Anemia, n (%) | 68 (56.7) | 36 (15.0) | <0.001* |
| Hypoalbuminemia, n (%) | 75 (62.5) | 39 (16.3) | <0.001* |
| Elevated serum creatinine, n (%) | 44 (36.7) | 26 (10.8) | <0.001* |
| HbA1c, % (mean ± SD_ | 8.0 ± 2.2 | 8.3 ± 2.8 | 0.27 |
P < 0.05. BMI, body mass index; CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; CTD, connective tissue disease; GC, glucocorticoid; IFD, invasive fungal disease; IQR, interquartile range; PSL, prednisolone; SD, standard deviation, T2DM, type 2 diabetes mellitus.
Multivariate regression analysis showed that anemia (adjusted OR 3.50, 95% CI 1.95–6.27, P < 0.001), hypoalbuminemia (adjusted OR 5.42, 95% CI 3.14–9.36, P < 0.001) and elevated serum creatinine (adjusted OR 2.08, 95% CI 1.07–4.04, P = 0.03) were associated factors for IFD in patients with type 2 diabetes (Table 6).
Table 6.
Factors associated with invasive fungal disease in type 2 diabetes patients
| Characteristics | Univariate logistic regression | Multivariate logistic regression | ||||
|---|---|---|---|---|---|---|
| Crude OR | 95% CI | P‐value | Adjusted OR | 95% CI | P‐value | |
| BMI (kg/m2) | 0.87 | 0.80–0.94 | <0.001* | – | – | – |
| Diabetic nephropathy | 2.39 | 1.42–4.03 | 0.001* | – | – | – |
| Lymphopenia | 4.93 | 2.70–8.99 | <0.001* | – | – | – |
| Anemia | 7.53 | 4.56–12.44 | <0.001* | 3.50 | 1.95–6.27 | <0.001* |
| Hypoalbuminemia | 9.02 | 5.45–14.92 | <0.001* | 5.42 | 3.14–9.36 | <0.001* |
| Elevated serum creatinine | 4.78 | 2.77–8.25 | <0.001* | 2.08 | 1.07–4.04 | 0.03* |
P < 0.05. BMI, body mass index; CI, confidence interval; IFD, invasive fungal disease; OR, odds ratio; T2DM, type 2 diabetes mellitus.
Discussion
In the present study, IFD was a life‐threatening complication in diabetes patients. C. albicans, C. neoformans and A. fumigatus were the major pathogens. Uncontrolled diabetes led to unfavorable outcomes. Anemia, hypoalbuminemia and elevated serum creatinine were associated with IFD in type 2 diabetes patients.
Yeast infection was the major infectious pattern in the present study. Diabetes, especially uncontrolled diabetes, provides a favorable environment for gas‐forming organisms, such as Candida spp., to grow22. Candiduria is the most common. A previous study showed that Candida spp. ranked second in the pathogens isolated in diabetes patients with urinary tract infection, after Escherichia coli 23. IAC is also frequently found. All the IAC occurred after gastrointestinal operation. Aside from the invasive procedure, patients with diabetes might bear a higher burden of intestinal C. albicans colonization24. It was found that the gut colony forming units of C. albicans in patients with type 1 diabetes is 2.7‐fold higher than their healthy counterparts25. Although frequently diagnosed, candidiasis was not the first ranking in the present study, which contradicts with previous report26. One of the explanations could be the exclusion of oral candidiasis, which is the most common27, vulvovaginal candidiasis and cutaneous candidiasis. Additionally, the variable definition of pulmonary candidiasis might yield different results. Candida spp. are part of the normal respiratory microflora28. Until recently, the diagnostic criteria for pulmonary candidiasis were obscure. In order to capture the real case, we applied a relatively strict inclusion criteria and only one patient with pathological evidence was diagnosed as pulmonary candidiasis. Therefore, the present results highlighted candiduria and IAC as the main infectious patterns of invasive candidiasis in patients with type 2 diabetes. Poor blood glucose control could worsen the situation.
Antifungal drug resistance is a matter of concern29. In the current study, the drug with the highest resistance rate was itraconazole, and more than one‐third of the C. glabrata was not sensitive to it. As the most frequently used drug, fluconazole is effective to treat most of the candidiasis, except C. tropicalis, whose resistance rate was 28.6%, similar to other reports (0–26.8%)30. In line with previous findings31, 32, amphotericin B or voriconazole resistance was very low in the present study. In the case of severe candidiasis, such as candidemia, amphotericin B or voriconazole is still recommended as the first‐line therapy21. Of note, the liposomal formulation of amphotericin B is found to be more effective in the eradication of the biofilm cells for all the Candida spp. than deoxycholate formulation33. Echinocandin tolerance was not detected in the present study. Echinocandin resistance is considered unusual. However, that of C. glabrata seemed to increase from 0% to 12.3% during the past two decades34, 35. In addition, caspofungin shows a higher biomass reduction capacity compared with micafungin, implying that caspofungin might be superior to micafungin in treating biofilm‐forming fungi36.
A high infective rate of cryptococcosis was observed in the present study. Diabetes was present in 8.5–33% of cryptococcosis cases in reported series37, 38, 39. Our results showed 16% of the type 2 diabetes patients with cryptococcosis had CTD. Cryptococcosis is a critical issue in patients with CTD. The incidence rate of cryptococcal meningitis in patients with systemic lupus erythematosus is 0.5%40. Approximately 0.2% of the patients with rheumatoid arthritis are complicated with cryptococcosis41. In addition, prolonged use of GC increases the risk of cryptococcosis42. Consistent with this, 20% of the type 2 diabetes patients with cryptococcosis received long‐term GC treatment in the present study, compared with 3.2% and 6.2% of patients with candidiasis and aspergillosis. Therefore, CTD and prolonged use of GC contribute to the high infective rate of cryptococcosis in type 2 diabetes.
Pulmonary mold infection is another major infectious pattern in type 2 diabetes patients. Different from previous findings43, mucormycosis was not common in the present study. The overall incidence of zygomycosis tends to decrease in patients with diabetes44. In addition, the involvement of diabetes in mucormycosis shows a large geographical variation. In Mexico, 72% of the patients with mucormycosis had diabetes44, but the number dropped to 17% in central Europe and Asia45. Furthermore, mucormycosis is likely to develop in patients with diabetic ketoacidosis, but just three patients with diabetic ketoacidosis were enrolled in our study45. Alternatively, Aspergillus spp. appeared to be the predominant pathogens. Antimicrobial susceptibility of Aspergillus spp. was not tested in our center. In previous research, the triazole resistance of A. fumigatus ranged from 0.6% to 29.6%30, and some isolates of A. flavus and A. ustus were resistant to amphotericin B46, 47, 48. The present results suggest that aspergillosis is common in type 2 diabetes patients without diabetic ketoacidosis, and drug resistance of Aspergillus spp. requires increasing attention.
Nosocomial IFD differs from community‐acquired IFD. Patients developing nosocomial IFD tended to be older and had longer disease duration. Candidiasis is common in nosocomial IFD, and this could be largely attributed to invasive procedures. Given that only a small number of patients had other underlying conditions, further analysis was not carried out. More information could be obtained in previous studies focusing on particular subpopulations49, 50, 51.
The present study was the first to explore co‐infection in diabetes patients with IFD. Nearly 48.3% of the patients had co‐infection, and mainly presented as pneumonia caused by Gram‐negative bacteria. Physicians should be vigilant against co‐infection in diabetes patients, even though IFD is established. Timely empiric coverage for Gram‐negative bacteria could improve the prognosis.
Several factors, including steroid use, neutropenia and immunosuppressants, are established risk factors for IFD. After adjusting these variables in multivariate logistic regression, we found anemia, hypoalbuminemia and elevated serum creatinine were associated with IFD in patients with type 2 diabetes. Anemia leads to poor outcomes in diabetes patients52. Half of the patients with IFD in the present study were aged >60 years. Age per se is a contributor to anemia, because the function of hematopoietic stem cells becomes inferior during aging53. Prolonged hyperglycemia, advanced glycated end‐products and the complication of diabetic nephropathy result in decreased production and/or impaired function of erythropoietin. In addition, chronic inflammation induced by hyperglycemia increases the consumption of hematopoietic materials54. Hypoalbuminemia is associated with infection and increases mortality55. Diabetes decreases the synthetic rate of serum albumin, and increases the transcapillary escape of albumin from the vascular to the interstitial compartment56, 57. Acute inflammation caused by infection suppresses the synthesis of albumin, and diabetic nephropathy increases albumin leakage through urine58. Therefore, early intervention of anemia and hypoalbuminemia is important to reduce the risk of IFD in patients with type 2 diabetes.
Inadequate blood glucose control is an independent risk factor for infection in diabetes patients59, 60. HbA1c levels were significantly higher in the deceased patients than survivors in the present study. However, as patients who require hospitalization usually have severe complications or suffer from uncontrolled blood glucose, the HbA1c levels did not differ between patients with and without IFD. The present results suggested that prolonged hyperglycemia leads to poor outcomes of IFD. Strict blood glucose control is still of great importance.
The present study had certain limitations. Data originated from one tertiary hospital in China. The generalization of the results to the type 2 diabetes population needs to be cautious. In addition, only type 2 diabetes patients were included. Whether the trends of IFD extend to patients with other diabetes subtypes remains to be elucidated. Additionally, although we tried to match some confounding factors, such as comorbidities between patients with and without IFD, no direct parameters, such as CD4+ lymphocyte count, were available to evaluate the immune status. Given the small number of patients involved in these medical backgrounds, we suggest studies on particular subpopulations would be more informative.
The present study provides an overview of IFD in patients with type 2 diabetes. Urinary candidiasis, pulmonary aspergillosis and pulmonary cryptococcosis are the main patterns. Aside from the traditional risk factors, anemia, hypoalbuminemia and elevated serum creatinine are associated with IFD in diabetes patients.
Acknowledgment
This project was supported by grants from the National Natural Science Foundation of China (81601403).
Disclosure
The authors declare no conflict of interest.
Appendix 1.
International Classification of Diseases 10th revision coding of type 2 diabetes and invasive fungal disease
| Disease | ICD‐10 coding |
|---|---|
| T2DM | E11.900, E11.800, E11.201+N08.3*, O24.300, E11.503, E11.301+H36.0*, E11.401+G63.2*, E13.900, E11.101, E11.406+G99.0*, E11.501+I79.2*, E11.302+H28.0*, E11.002, E11.601+M14.2*, E11.001, E11.505, E11.303+H22.1*, E11.405+G73.0*, E11.504, E11.603+L99.8*, E11.102, E11.403+G63.2*, E11.404+G99.0*, E11.103, E11.003, E11.502+I79.2*, E11.402+G99.0*, E11.700, E11.000, E11.600, E11.200, E11.400, E11.100, E11.300, E11.604, E13.800, E14.900, E14.800, E13.700, E14.000, E13.000, E14.600, E13.600, E14.200, E13.200, E14.400, E13.400, E14.100, E13.100, E14.300, E13.300, E14.500, E13.500, O24.100 |
| Aspergillosis | B44.051+, B44.101+, B44.102+, B44.103+, B44.151, B44.751, B44.752, B44.801, B44.901 |
| Blastomycosis | B40, B40.051+, B40.151+, B40.201+, B40.751, B40.752, B40.851, B40.901 |
| Candidiasis | B37.101+, B37.401+, B37.551+, B37.601+, B37.751, B37.801, B37.802, B37.803, B37.804, B37.805, B37.806+, B37.81, B37.852 |
| Coccidioidomycosis | B38, B38.051+, B38.052+, B38.151+, B38.201+, B38.451+, B38.751, B38.752, B38.851, B38.901, B38.051+ |
| Cryptococcosis | B45.001+, B45.101+, B45.102+, B45.103+, B45.351+, B45.751, B45.752, B45.851, B45.901 |
| Fungal disease | B49.X51 |
| Histoplasmosis | B39.051+, B39.151+, B39.201+, B39.251+, B39.352+, B39.353, B39.451, B39.901 |
| Mucormycosis | B46.001+, B46.151+, B46.251+, B46.451, B46.452, B46.501 |
| Mycosis | B48.751, B49.XO1, B49.XO2, B49.XO4+, B49.XO5, B49.XO6 B49.XO7+, B49.XO9+, B49.X10+, B49.X11, B49.X12+, B49.X13+, B49.X14+, B49.X15, B49.X16+ |
| Paracoccidioidomycosis | B41.700, B41.900, B41.800, B41.000 |
| Penicilliosis | B48.451 |
| Sporotrichosis | B42, B42.151, B42.751, B42.152, B42.851, B42.901 |
| Zygomycosis | B46.952 |
ICD, International Classification of Diseases; IFD, invasive fungal disease; T2DM, type 2 diabetes mellitus.
Appendix 2.
Criteria for diagnosis of International Classification of Diseases in patients with type 2 diabetes
| Category | Criteria |
|---|---|
| Proven | Histopathological examination reveals fungal infection in normally sterile sites, or recovery of a mold/yeast from samples obtained by a sterile procedure, or blood culture yielding a mold/yeast (Aspergillus spp. excluded) |
| Probable | Patients with DM satisfying the following clinical and mycological criteria were considered to have probable mold infection. |
| Mold infection | a. Clinical criteria |
| Lung infection: pulmonary CT scan showed (i) dense, well‐circumscribed lesions with or without a halo sign, or (ii) air‐crescent sign, or (iii) cavity; or bronchoscopy found tracheobronchial ulceration, nodule, pseudomembrane, plaque or escha. | |
| Sinonasal infection: imaging suggesting sinusitis with (i) acute localized pain, and/or (ii) evidence of bone erosion, and/or (iii) nasal ulcer with black eschar revealed by nasal endoscopy | |
| CNS infection: imaging showing focal lesions or meningeal enhancement | |
| b. Mycological criteria: satisfying at least one of the following criteria | |
| (i) Mold in sputum, BALF, bronchial brush or sinus aspirate samples | |
| (ii) Positive GM antigen detection in plasma, serum, BALF or CSF (for aspergillosis only). | |
| Cryptococcosis | Patients with DM showing one of the aforementioned radiographic manifestation along with (i) Cryptococcus spp. isolated in sputum, BALF, bronchial brush; or (ii) positivie CrAg detection in serum or CSF. |
| Possible | |
| Mold infection/cryptococcosis | Patients with DM satisfying the aforementioned clinical criteria, but without mycological evidence were considered to have possible mold infection/cryptococcosis. |
| Yeast infection | a. Lung infection: patients with DM satisfying all the following criteria were considered to have possible pulmonary candidiasis: (i) clinical symptoms suggesting lung infection and CT scan showing new onset bronchopneumonia or micronodules or diffused infiltrates, (ii) positive twice in microscopic examination showing fungal hyphae/pseudohyphae and recovery twice of the same yeast in sputum/BALF culture, (iii) positive twice in serum G‐test, (iv) excluded other possible pathogens |
| b. UTI: patients with DM satisfying all the following criteria were considered to have possible urinary candidiasis: (i) clinical symptoms indicating urinary infection, (ii) increased leukocytes in clean‐catch midstream urine, (iii) positive twice in urine culture for the same yeast, (iv) excluded other possible pathogens | |
| c. Esophageal infection: patients with DM satisfying all the following criteria were considered to have possible esophageal candidiasis: (i) white plaques found in endoscopic examination, (ii) fungal hyphae, pseudohyphae or spores found in esophageal brushing sample | |
BALF, bronchoalveolar lavage fluid; CNS, central nervous system; CrAg, cryptococcal capsule polyglycan antigen; CSF, cerebrospinal fluid; CT, computed tomography; DM, diabetes mellitus; G‐test, β‐D‐glucan detection; GM, galactomannan; IFD, invasive fungal disease; T2DM, type 2 diabetes mellitus; UTI, urinary tract infection.
J Diabetes Investig 2020; 11: 731–744
References
- 1. Muller LM, Gorter KJ, Hak E, et al Increased risk of common infections in patients with type 1 and type 2 diabetes mellitus. Clin Infect Dis 2005; 41: 281–288. [DOI] [PubMed] [Google Scholar]
- 2. Korbel L, Spencer JD. Diabetes mellitus and infection: an evaluation of hospital utilization and management costs in the United States. J Diabetes Complications 2015; 29: 192–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gregg EW, Cheng YJ, Srinivasan M, et al Trends in cause‐specific mortality among adults with and without diagnosed diabetes in the USA: an epidemiological analysis of linked national survey and vital statistics data. Lancet 2018; 391: 2430–2440. [DOI] [PubMed] [Google Scholar]
- 4. Alegre‐Díaz J, Herrington W, López‐Cervantes M, et al Diabetes and cause‐specific mortality in Mexico City. N Engl J Med 2016; 375: 1961–1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bassetti M, Garnacho‐Montero J, Calandra T, et al Intensive care medicine research agenda on invasive fungal infection in critically ill patients. Intensive Care Med 2017; 43: 1225–1238. [DOI] [PubMed] [Google Scholar]
- 6. Trof RJ, Beishuizen A, Debets‐Ossenkopp YJ, et al Management of invasive pulmonary aspergillosis in non‐neutropenic critically ill patients. Intensive Care Med 2007; 33: 1694–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Li Y, Fang W, Jiang W, et al Cryptococcosis in patients with diabetes mellitus II in mainland China: 1993–2015. Mycoses 2017; 60: 706–713. [DOI] [PubMed] [Google Scholar]
- 8. Nunes JO, Pillon KR, Bizerra PL, et al The simultaneous occurrence of histoplasmosis and cryptococcal fungemia: a case report and review of the literature. Mycopathologia 2016; 181: 891–897. [DOI] [PubMed] [Google Scholar]
- 9. Cataldi V, Di Campli E, Fazii P, et al Candida species isolated from different body sites and their antifungal susceptibility pattern: cross‐analysis of Candida albicans and Candida glabrata biofilms. Med Mycol 2017; 55: 624–634. [DOI] [PubMed] [Google Scholar]
- 10. Rodrigues CF, Henriques M. Portrait of matrix gene expression in Candida glabrata biofilms with stress induced by different drugs. Genes (Basel) 2018; 9: 205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Iñigo M, Del Pozo JL. Fungal biofilms: from bench to bedside. Rev Esp Quimioter 2018; 31(Suppl 1): 35–38. [PMC free article] [PubMed] [Google Scholar]
- 12. Alsahhaf A, Al‐Aali KA, Alshagroud RS, et al Comparison of yeast species in the subgingival oral biofilm of individuals with type 2 diabetes and peri‐implantitis and individuals with peri‐implantitis without diabetes. J Periodontol 2019; 90: 1383–1389. [DOI] [PubMed] [Google Scholar]
- 13. Gulia S, Bhatt V, Shetty M, et al Effect of type II diabetes Mellitus, Candida albicans and Streptococcus mutans on the biofilm formation on prosthetic materials. J Contemp Dent Pract 2018; 19: 1538–1545. [PubMed] [Google Scholar]
- 14. Chan JC, Zhang Y, Ning G. Diabetes in China: a societal solution for a personal challenge. Lancet Diabetes Endocrinol 2014; 2: 969–979. [DOI] [PubMed] [Google Scholar]
- 15. Chen M, Xu Y, Hong N, et al Epidemiology of fungal infections in China. Front Med 2018; 12: 58–75. [DOI] [PubMed] [Google Scholar]
- 16. World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 2013; 310: 2191–2194. [DOI] [PubMed] [Google Scholar]
- 17. Chinese Medical Association . Chinese guideline for type 2 diabetes mellitus. Chin J Diabetes 2014; 22: 2–42 (Chinese). [Google Scholar]
- 18. Lewis G, Maxwell AP. Risk factor control is key in diabetic nephropathy. Practitioner 2014; 258(13–7): 2. [PubMed] [Google Scholar]
- 19. Ascioglu S, Rex JH, de Pauw B, et al Defining opportunistic invasive fungal infections in immunocompromised patients with cancer and hematopoietic stem cell transplants: an international consensus. Clin Infect Dis 2002; 34: 7–14. [DOI] [PubMed] [Google Scholar]
- 20. De Pauw B, Walsh TJ, Donnelly JP, et al Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses. Clin Infect Dis 2008; 46: 1813–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Scientific Working Group . The diagnosis and treatment of Candidiasis: the expert consensus. Clin J Infect Chemother 2011; 11: 81–95 (Chinese). [Google Scholar]
- 22. Huang JJ, Tseng CC. Emphysematous pyelonephritis: Clinicoradiological classification, management, prognosis, and pathogenesis. Arch Intern Med 2000; 160: 797–805. [DOI] [PubMed] [Google Scholar]
- 23. Papazafiropoulou A, Daniil I, Sotiropoulos A, et al Urinary tract infection, uropathogens and antimicrobial resistance in diabetic and nondiabetes patients. Diabetes Res Clin Pract 2009; 85: e12–13. [DOI] [PubMed] [Google Scholar]
- 24. Gürsoy S, Koçkar T, Atik SU, et al Autoimmunity and intestinal colonization by Candida albicans in patients with type 1 diabetes at the time of the diagnosis. Korean J Pediatr 2018; 61: 217–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Soyucen E, Gulcan A, Aktuglu‐Zeybek AC, et al Differences in the gut microbiota of healthy children and those with type 1 diabetes. Pediatr Int 2014; 56: 336–343. [DOI] [PubMed] [Google Scholar]
- 26. Rodrigues CF, Rodrigues ME, Henriques M. Candida sp. Infections in patients with diabetes mellitus. J Clin Med 2019; 8: E76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Akpan A, Morgan R. Oral candidiasis. Postgrad Med J 2002; 78: 455–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pendleton KM, Huffnagle GB, Dickson RP. The significance of Candida in the human respiratory tract: our evolving understanding. Pathog Dis 2017; 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bougnoux ME, Brun S, Zahar JR. Healthcare‐associated fungal outbreaks: new and uncommon species, new molecular tools for investigation and prevention. Antimicrob Resist Infect Control 2018; 7: 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gonçalves SS, Souza ACR, Chowdhary A, et al Epidemiology and molecular mechanisms of antifungal resistance in Candida and Aspergillus. Mycoses 2016; 59: 198–219. [DOI] [PubMed] [Google Scholar]
- 31. da Matta DA, de Almeida LP, Machado AM, et al Antifungal susceptibility of 1000 Candida bloodstream isolates to 5 antifungal drugs: results of a multicenter study conducted in Sao Paulo, Brazil, 1995–2003. Diagn Microbiol Infect Dis 2007; 57: 399–404. [DOI] [PubMed] [Google Scholar]
- 32. Hull CM, Parker JE, Bader O, et al Facultative sterol uptake in an ergosterol‐deficient clinical isolate of Candida glabrata harboring a missense mutation in ERG11 and exhibiting cross‐resistance to azoles and amphotericin B. Antimicrob Agents Chemother 2012; 56: 4223–4232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rodrigues CF, Henriques M Liposomal and deoxycholate Amphotericin B formulations: effectiveness against biofilm infections of Candida spp. Pathogens 2017; 6: E62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Alexander BD, Johnson MD, Pfeiffer CD, et al Increasing echinocandin resistance in Candida glabrata: clinical failure correlates with presence of FKS mutations and elevated minimum inhibitory concentrations. Clin Infect Dis 2013; 56: 1724–1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Arendrup MC, Perlin DS. Echinocandin resistance: an emerging clinical problem? Curr Opin Infect Dis 2014; 27: 484–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rodrigues CF, Rodrigues ME, Henriques M. Susceptibility of Candida glabrata biofilms to echinocandins: alterations in the matrix composition. Biofouling 2018; 34: 569–578. [DOI] [PubMed] [Google Scholar]
- 37. Kiertiburanakul S, Wirojtananugoon S, Pracharktam R, et al Cryptococcosis in human immunodeficiency virus‐negative patients. Int J Infect Dis 2006; 10: 72–78. [DOI] [PubMed] [Google Scholar]
- 38. Shih CC, Chen YC, Chang SC. Cryptococcal meningitis in non‐HIV‐infected patients. QJM 2000; 93: 245–251. [DOI] [PubMed] [Google Scholar]
- 39. Tseng HK, Liu CP, Ho MW, et al Microbiological, epidemiological, and clinical characteristics and outcomes of patients with cryptococcosis in Taiwan, 1997–2010. PLoS ONE 2013; 8: e61921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fang W, Chen M, Liu J, et al Cryptococcal meningitis in systemic lupus erythematosus patients: pooled analysis and systematic review. Emerg Microbes Infect 2016; 5: e95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Liao TL, Chen YM, Chen DY. Risk factors for cryptococcal infection among patients with rheumatoid arthritis receiving different immunosuppressive medications. Clin Microbiol Infect 2016; 22: 815.e811–815.e813. [DOI] [PubMed] [Google Scholar]
- 42. Wang LR, Barber CE, Johnson AS, et al Invasive fungal disease in systemic lupus erythematosus: a systematic review of disease characteristics, risk factors, and prognosis. Semin Arthritis Rheum 2014; 44: 325–330. [DOI] [PubMed] [Google Scholar]
- 43. Corzo‐León DE, Chora‐Hernández LD, Rodríguez‐Zulueta AP, et al Diabetes mellitus as the major risk factor for mucormycosis in Mexico: Epidemiology, diagnosis, and outcomes of reported cases. Med Mycol 2018; 56: 29–43. [DOI] [PubMed] [Google Scholar]
- 44. Roden MM, Zaoutis TE, Buchanan WL, et al Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin Infect Dis 2005; 41: 634–653. [DOI] [PubMed] [Google Scholar]
- 45. Rüping MJ, Heinz WJ, Kindo AJ, et al Forty‐one recent cases of invasive zygomycosis from a global clinical registry. J Antimicrob Chemother 2010; 65: 296–302. [DOI] [PubMed] [Google Scholar]
- 46. Azzola A, Passweg JR, Habicht JM, et al Use of lung resection and voriconazole for successful treatment of invasive pulmonary Aspergillus ustus infection. J Clin Microbiol 2004; 42: 4805–4808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Goncalves SS, Stchigel AM, Cano J, et al In vitro antifungal susceptibility of clinically relevant species belonging to Aspergillus section Flavi. Antimicrob Agents Chemother 2013; 57: 1944–1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Shivaprakash MR, Geertsen E, Chakrabarti A, et al In vitro susceptibility of 188 clinical and environmental isolates of Aspergillus flavus for the new triazole isavuconazole and seven other antifungal drugs. Mycoses 2011; 54: e583–539. [DOI] [PubMed] [Google Scholar]
- 49. Lao M, Zhan Z, Su F, et al Invasive mycoses in patients with connective tissue disease from Southern China: clinical features and associated factors. Arthritis Res Ther 2019; 21: 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Pergam SA. Fungal pneumonia in patients with hematologic malignancies and hematopoietic cell transplantation. Clin Chest Med 2017; 38: 279–294. [DOI] [PubMed] [Google Scholar]
- 51. Fracchiolla NS, Mancini V, Nosari A, et al Changes in the incidence of candidemia and related mortality in patients with hematologic malignancies in the last ten years. A SEIFEM 2015‐B report. Haematologica 2017; 102: e407–e410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Valera FC, do Lago T, Tamashiro E, et al Prognosis of acute invasive fungal rhinosinusitis related to underlying disease. Int J Infect Dis 2011; 15: e841–844. [DOI] [PubMed] [Google Scholar]
- 53. Groarke EM, Young NS. Aging and Hematopoiesis. Clin Geriatr Med 2019; 35: 285–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Sahay M, Kalra S, Badani R, et al Diabetes and Anemia: International Diabetes Federation (IDF) ‐ Southeast Asian Region (SEAR) position statement. Diabetes Metab Syndr 2017; 11(Suppl 2): S685–S695. [DOI] [PubMed] [Google Scholar]
- 55. Peng L, Xu Z, Huo Z, et al New insights into the clinical characteristics and prognostic factors of pulmonary fungal infections from a retrospective study in Southwestern China. Infect Drug Resist 2018; 11: 307–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Nicholson JP, Wolmarans MR, Park GR. The role of albumin in critical illness. Br J Anaesth 2000; 85: 599–610. [DOI] [PubMed] [Google Scholar]
- 57. Boldt J. Use of albumin: an update. Br J Anaesth 2010; 104: 276–284. [DOI] [PubMed] [Google Scholar]
- 58. Moshage HJ, Janssen J, Franssen JH, et al Study of the molecular mechanism of decreased liver synthesis of albumin in inflammation. J Clin Invest 1987; 79: 1635–1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Davis KL, Wei W, Meyers JL, et al Association between different Hemoglobin A1c levels and clinical outcomes among elderly nursing home residents with type 2 diabetes mellitus. J Am Med Dir Assoc 2014; 15: 757–762. [DOI] [PubMed] [Google Scholar]
- 60. Pearson‐Stuttard J, Blundell S, Harris T, et al Diabetes and infection: assessing the association with glycaemic control in population‐based studies. Lancet Diabetes Endocrinol 2016; 4: 148–158. [DOI] [PubMed] [Google Scholar]


