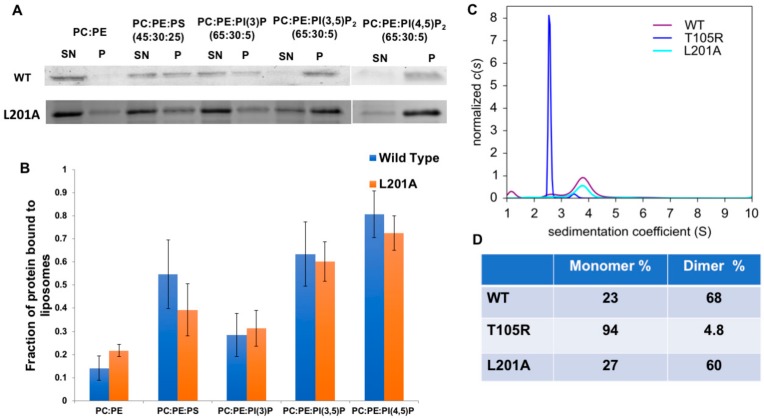
Figure 5.
Biophysical properties of the L201A mutant are similar to WT mVP40 including anionic membrane binding properties and dimeric state in solution. (A) SDS-PAGE of supernatant (SN) and pellet (P) fractions collected from MLV sedimentation assays on lipid compositions and ratios appearing above the images for WT and L201A proteins. (B) Quantification of MLV sedimentation assays. The data represent the average of three sedimentation assays with error bars representing the standard error of the mean. (C) mVP40 protein size distribution was obtained through fitting data from sedimentation velocity experiments to continuous distribution c(s) Lamm equation model. (D) Monomer and dimer percentages of WT mVP40 (WT), T105R and L201A mutants obtained from sedimentation velocity experiments.