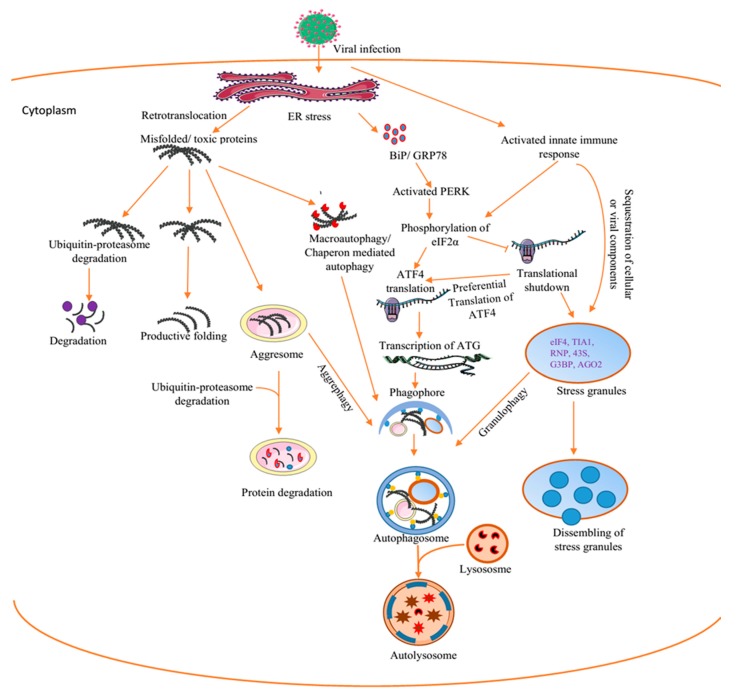
Figure 2.
Interplay between virus-induced cellular aggregates and inclusions. The replication of viruses triggers ER stress. This causes the release of BiP/GRP79 into the cytoplasm and the subsequent activation of the unfolded protein response (UPR). This could ultimately lead to the clearance of toxic proteins by autophagy. Alternatively, virus infection directly activates innate immune responses capable of causing translational shutdown and stress granules (SGs) formation. Cellular and or viral proteins could appear as misfolded or toxic proteins and are recruited to the chaperon pathway for productive refolding and/or to the proteasomal pathway. However, when these pathways are blocked, the misfolded proteins are sequestrated in the aggresomes and are cleared by autophagy–aggrephagy. BiP/GRP78, binding immunoglobulin protein/glucose-regulated protein 78, ATF4, activating transcription factor 4, eIF2α, eukaryotic initiation factor 2 alpha, ATG, autophagy-related protein, RNP, ribronucleoprotein, AGO2aArgonaute, G3BP-1, Ras GTPase-activating protein-binding protein-1, PERK, protein kinase-like endoplasmic reticulum kinase. Figure was drawn on smart.servier.com.