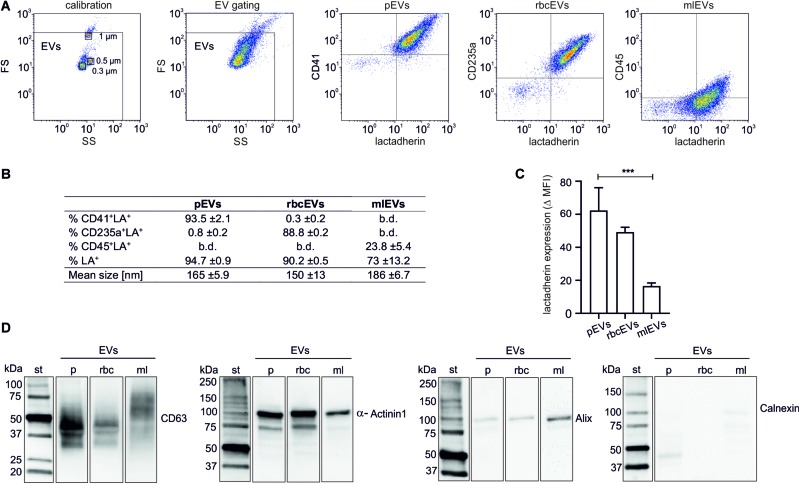
FIGURE 1.
Characterization of extracellular vesicle fractions. EVs were isolated from platelet concentrates (pEVs), red blood cell concentrates (rbcEVs), or from monocytic THP-1 cells (mlEVs). Characterization of isolated EV fractions was performed using flow cytometry, nanoparticle tracking analysis, and Western Blotting, as described in the section “Materials and Methods.” (A) Flow cytometric characterization of EVs was performed after calibration with fluorescent silica beads. EVs were defined as phosphatidylserine-exposing (i.e., lactadherin-positive) events in the EV gate, and their cellular origin was assessed using CD41, CD235a, and CD45 as markers for pEVs, rbcEVs, and mlEVs, respectively. (B) Exposure of markers of cellular origin (n = 10) and mean size of pEVs, rbcEVs, and mlEVs (n = 11); b.d., below detection. (C) Difference between the specific antibody staining and the respective fluorochrome-labeled reagent control (median fluorescence intensity, MFI) for lactadherin staining. Significance (***p ≤ 0.001) was calculated for pEVs vs. mlEVs (n = 10); as only two batches of rbcEVs were available, significance cannot be indicated for rbcEVs vs. mlEVs. (D) Characterization of pEVs, rbcEVs, and mlEVs using Western blotting (10 μg of protein per lane). The blot images are presented as grouped images from different parts of the same membrane or from different membranes. The original blots are available online as Supplementary Datasheet S2 of the Supplementary Material. b.d., below the limit of detection.