FIGURE 1.
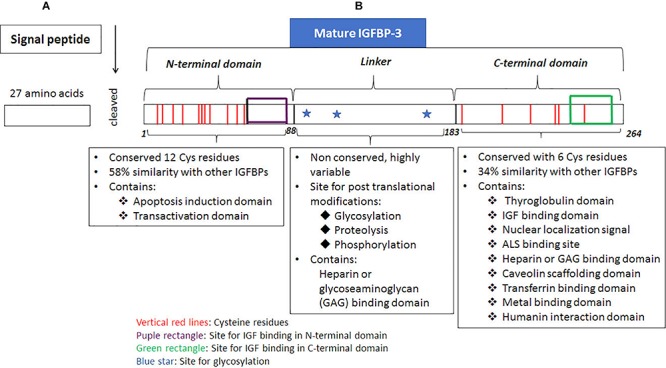
Structure of IGFBP-3. Translation is initiated on the ribosomes, (A) signal sequence, a 27 amino acid region present on the N-terminal end of the nascent IGFBP-3 polypeptide chain is responsible for targetting it onto the surface of rough endoplasmic, which is recognized by the signal recognition particle (SRP). Through the concerted action of two G-proteins, namely SRP that binds to the signal sequence; and SRP receptor that is present on the endoplasmic reticulum (ER), the nascent polypeptide chain with the signal sequence binds onto a channel protein, translocon along with the ribosome, mRNA. The nascent poplypeptide chain of IGFBP-3 is translated on ER and through the translocon moves into the lumen of ER where the signal sqence is excised by proteases. (B) Mature IGFBP-3 consists of N-terminal domain, linker domain and the C-terminal domain. Posttranslational modification of glycosylation occurs in the lumen of ER and Golgi complex in the mid-linker domain of IGFBP-3. Various domains of IGFBP-3 protein and their associated functions are dipicted in the figure.
