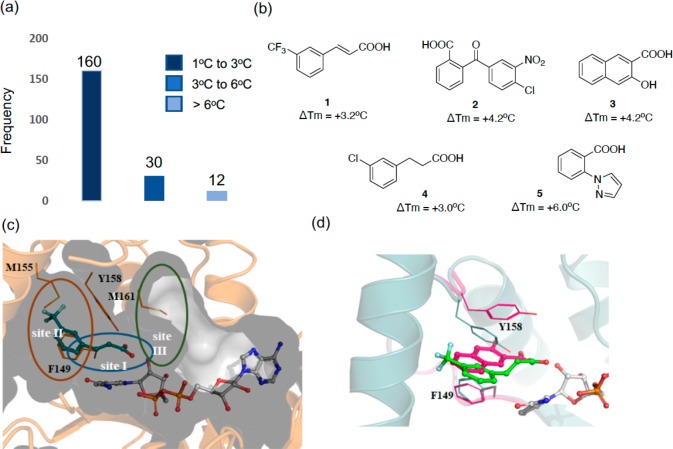
Figure 1.
(a) Histogram of fragments (5 mM) found to increase the melting point (ΔTm) of InhA (10 μM) in the presence of NAD+ (1 mM) by a fluorescence-based thermal-shift assay. (b) Chemical structures of the X-ray hits obtained. (c) X-ray structure of fragment 1 (teal) bound in an InhA–NAD+ complex (gray), showing the cavity surface. The substrate binding regions of InhA are divided into three sites: site I, site II, and site III. Fragment 1 occupies the catalytic site I and a part of the hydrophobic pocket site II, and Y158 adopts an “in” conformation. (d) Overlaid X-ray crystal structures of fragments 1 (green) and 3 (magenta) binding to InhA in the presence of NAD+ with Y158 in an open conformation for fragment 1 (green, PDB Code 6SQ5) and in a new, previously unseen, conformation for fragment 3 (magenta, PDB Code 6SQ9).