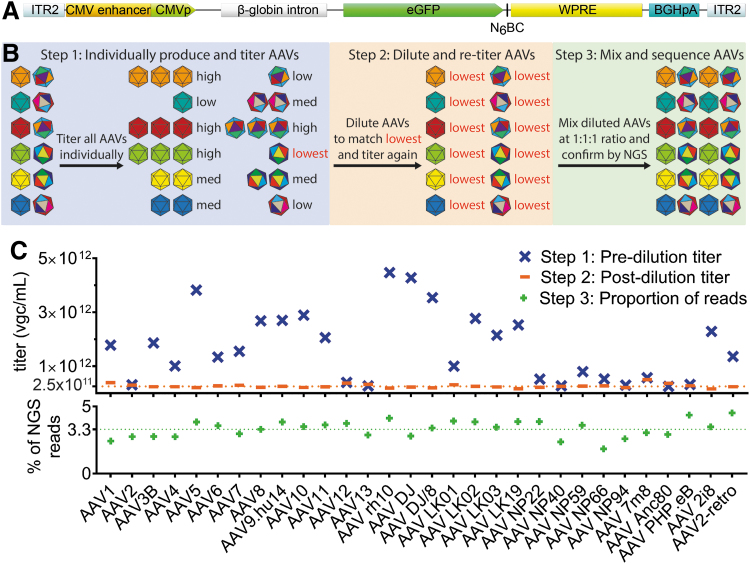
Figure 1.
Experimental setup and preparation of the AAV Testing Kit mix. (A) The ITR2-CMVp-eGFP-N6BC-WPRE-ITR2 transgene packaged in the individual AAV vectors used in the study. (B) Individual steps involved in the preparation of the AAV Testing Kit. (C) Upper panel: titration of individually produced AAVs (Step 1) and diluted AAV variants (Step 2). The dilution benchmark of 2.5 × 1011 vgc/mL is indicated by the orange dotted line. Lower panel: Results of the NGS analysis of the AAV distribution in the AAV Testing Kit used in this study. The expected % contribution of individual variants (100% ÷ 30 = ∼3.33%) is indicated by the green dotted line. AAV, adeno-associated virus; N6BC, unique 6 nucleotide barcode; NGS, next-generation sequencing.