Figure 3:
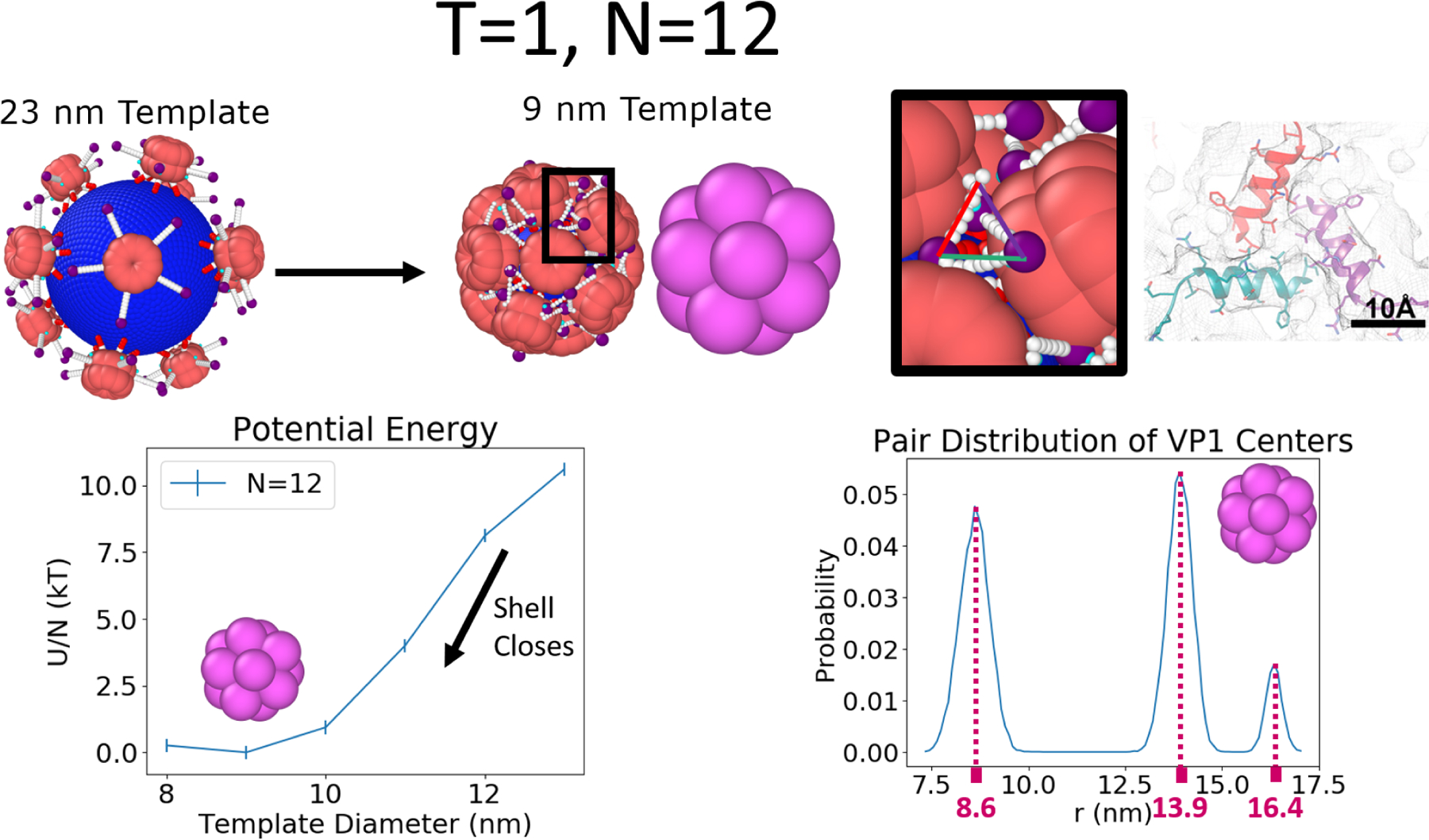
The T = 1 capsid is made of N=12 VP1 pentamers. The relative potential energy per VP1 and pairwise distance distribution of the capsid as a function of the template diameter is measured by first allowing 12 VP1 pentamers to bind to a large template and then slowly reducing the size of the capsid such that no VP1 pentamers are released during this process illustrated at the top left of the figure. Results on the bottom left show that the minimum of the potential energy occurs at a template diameter of 9 nm. On the bottom right, we see 3 peaks matching the icosahedral symmetry (see main text) and large regions of zero probability, indicating the static nature of the VP1 pentamers in this configuration. Since this static T = 1 configuration is stable over many template sizes we believe that it would also be robust against changes in template size based on changes in salt, pH, etc., provided that these changes do not impact the VP1-VP1 or VP1-template interactions too much. This minimum energy structure also recovers the presence of a three helix triangle located at the three fold symmetry points of the icosahedron,15 shown at the top right. The image of the three-helix triangle with the scale bar was adapted with permission from Kler et al., ACS Chemical Biology 2013, 8, 2753–2761. Copyright 2013 American Chemical Society.
