Figure 1.
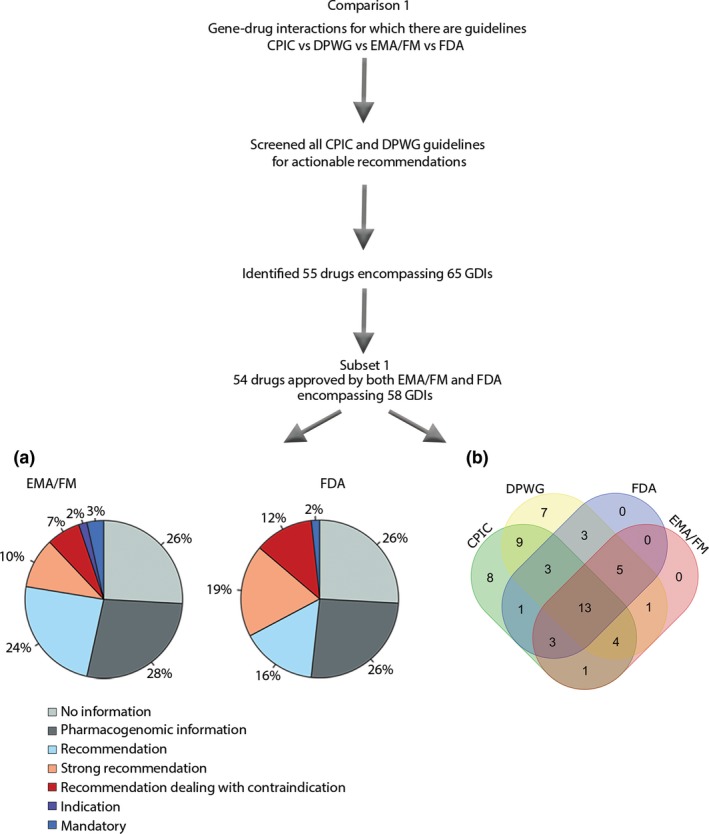
Selection of drugs for comparison 1, comparing CPIC, DPWG, EMA/FM, and FDA, was based on all CPIC and DPWG guidelines that contain recommendations (55 drugs with 65 GDIs). The distribution of label categories was compared in a subset of these GDIs (subset 1), only including those that are approved by both the EMA/FM and the FDA, leaving 58 GDIs. (a) Pie charts showing the distribution of different label categories between the EMA/FM and the FDA for 58 GDIs that have guidelines by the CPIC and/or DPWG. (b) Venn diagram of the same 58 GDIs that have guidelines by the CPIC and/or DPWG and are approved by both the EMA/FM and the FDA, with actionable labels (mandatory, indication, recommendation dealing with contraindication, or (strong) recommendation). For 13 of the GDIs (abacavir, allopurinol, atomoxetine, azathioprine (2 genes), capecitabine, carbamazepine, citalopram, codeine, mercaptopurine (2 genes), and thioguanine (2 genes)) there are common guidelines or actionable labels by all consortia and agencies. For 9 (15.5%) of the GDIs (clomipramine – CYP2D6, doxepin, imipramine (2 genes), nortriptyline, paroxetine, simvastatin, tacrolimus, and voriconazole) guidelines are provided by both the CPIC and DPWG but no actionable labels by the EMA/FM or the FDA. A further eight (13.8%) and seven (12.1%) GDIs that have guidelines by the CPIC or DPWG, respectively, are unaccounted for in both the EMA/FM and the FDA SmPCs (atazanavir, atorvastatin, clomipramine, doxepin, efavirenz, flecainide, lamotrigine, metoprolol, ondansetron, peginterferon, ribavirin, tamoxifen, and trimipramine (2 genes) and venlafaxine). CPIC, Clinical Pharmacogenetics Implementation Consortium; DPWG, Dutch Pharmacogenetics Working Group; EMA/FM, European Medicines Agency/FIDMD+MEB; FDA, US Food and Drug Administration; GDI, gene–drug interaction.
