Ablation of the mitochondrial protein Efhd1 in mice interferes with normal development of sensory axons and results in mitochondrial dysfunction associated with activation of cellular stress pathways.
Abstract
During development, neurons adjust their energy balance to meet the high demands of robust axonal growth and branching. The mechanisms that regulate this tuning are largely unknown. Here, we show that sensory neurons lacking liver kinase B1 (Lkb1), a master regulator of energy homeostasis, exhibit impaired axonal growth and branching. Biochemical analysis of these neurons revealed reduction in axonal ATP levels, whereas transcriptome analysis uncovered down-regulation of Efhd1 (EF-hand domain family member D1), a mitochondrial Ca2+-binding protein. Genetic ablation of Efhd1 in mice resulted in reduced axonal morphogenesis as well as enhanced neuronal death. Strikingly, this ablation causes mitochondrial dysfunction and a decrease in axonal ATP levels. Moreover, Efhd1 KO sensory neurons display shortened mitochondria at the axonal growth cones, activation of the AMP-activated protein kinase (AMPK)–Ulk (Unc-51–like autophagy-activating kinase 1) pathway and an increase in autophagic flux. Overall, this work uncovers a new mitochondrial regulator that is required for axonal morphogenesis.
Introduction
During development, neurons grow axons that elongate over substantial distances and branch for proper tissue innervation (Vaarmann et al, 2016). In humans, the axons of the peripheral nervous system (PNS) can reach lengths of up to 1 m (Misgeld & Schwarz, 2017). As axonal morphogenesis is energetically demanding, it must be supported by a tightly regulated energy balance.
Axonal ATP is produced primarily in the mitochondria, which are predominately localized in metabolically active zones of the neuron such as the growth cones at the leading edge of the axon (Vaarmann et al, 2016; Sheng, 2017). Mitochondrial function is critical to axonal morphogenesis; numerous reports have demonstrated that mitochondrial biogenesis, localization, trafficking, and local ATP production are all limiting factors for axonal growth and morphogenesis (Courchet et al, 2013; Spillane et al, 2013; Vaarmann et al, 2016; Misgeld & Schwarz, 2017). However, the regulatory mechanisms that couple axonal morphogenesis and energy supply remain poorly understood. The tumor-suppressor protein liver kinase B1 (Lkb1, also called Stk11) is a well-known regulator of cellular polarization in epithelia (Hardie, 2007; Shackelford & Shaw, 2009) and other nonneural tissues in Drosophila and vertebrates (Nakano & Takashima, 2012). In addition, studies in nonneuronal cells have established a critical function of the Lkb1 pathway in energy homeostasis mediated through enhancement of mitochondrial activity, mitochondrial biogenesis, and autophagy, as well as via a mammalian target of rapamycin-dependent decrease in energy expenditure and protein synthesis (Alexander & Walker, 2011; Hardie, 2011). Studies of the neuronal function of Lkb1 in the central nervous system (CNS) initially revealed its key role in establishing axon polarization and extension through the activation of the synapses of amphids defective kinases (Barnes et al, 2007; Shelly et al, 2007). More recently, deletion of Lkb1 in the CNS revealed that it also contributes to axonal morphogenesis, in part through its effect on mitochondrial movement, biogenesis, and localization (Courchet et al, 2013; Spillane et al, 2013).
This study reports the discovery of a new pathway that couples energy homeostasis to axonal growth. In our investigation, we ablated the Lkb1 gene in mice at the onset of PNS development. Lkb1-KO animals exhibited abnormal axonal growth and branching and reduced axonal ATP production. Intriguingly, transcriptome analysis of Lkb1 KO sensory neurons uncovered significant down-regulation of the RNA transcript of the mitochondrial protein EF-hand domain family member D1 (Efhd1, also known as mitocalcin). Efhd1 is a calcium-binding protein that is localized to the inner mitochondrial membrane (Tominaga et al, 2006). To explore the function of Efhd1 in sensory neurons, we generated an Efhd1 KO mouse line. Herein, we characterize these animals and demonstrate that Efhd1 regulates mitochondrial function and axonal morphogenesis during PNS development, providing a novel link of mitochondrial activity and energy homeostasis to axonal morphogenesis.
Results
Lkb1 KO sensory neurons display normal polarization but reduced axonal growth in vitro
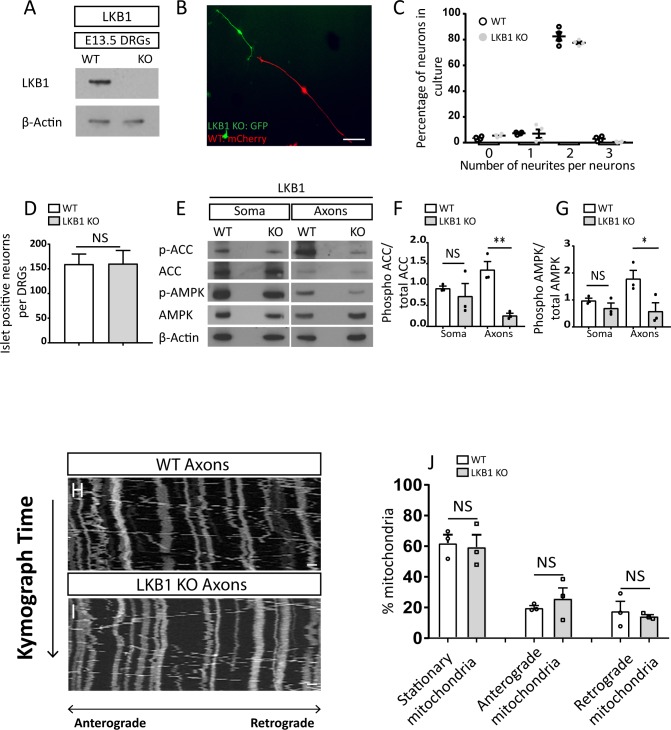
To test the function of Lkb1 in the development of the PNS, we ablated the floxed Lkb1 gene in the mouse at embryonic day 9 (E9) using the Wnt1–cre line, generating the strain henceforth referred to as Lkb1 KO (Swisa et al, 2015) (Fig S1A). We first tested the polarization of dorsal root ganglion (DRG) neurons in vitro. After transfecting WT and Lkb1 KO neurons with mCherry- and GFP-expressing plasmids, respectively, we cocultured the differentially labeled cells. This approach eliminates any effects that may arise from technical variations between the cultures or non-cell autonomous effects (such as secreted factors). Dissociated DRG neurons at E12.5 typically exhibit polarized morphology with a pair of axons growing from two opposite sides of the soma (Tymanskyj et al, 2018). Analysis of the Lkb1 KO and WT neurons established that after 48 h, both cell types exhibit normal polarized morphology, with two axonal branches sprouting from opposite sides of the cell body (Fig S1B and C). These results support the conclusion of a previous study that suggested Lkb1 is dispensable for axon formation/polarization outside of the cortex (Lilley et al, 2013).
Figure S1. Liver kinase B1 (Lkb1) ablation by Wnt1–cre causes metabolic abnormalities.
(A) Immunoblot of WT and Lkb1 KO of dorsal root ganglions (DRGs) from E13.5 embryos. (B) Mixed culture of WT and Lkb1 KO DRGs from E12.5 embryos transfected with two different plasmids, WT: mCherry, Lkb1 KO: GFP. Neurons were fixed and directly visualized after 48 h. Scale bar 500 μm. (C) Quantification of number of neurites per cells was analyzed. Graph shows means ± SEM based on four independent experiments (N = 4), 30 neurons in each experiment were analyzed (unpaired t test NS). (D) Quantification of Islet1 positive-neurons in E15.5 WT and Lkb1 KO DRGs. Six WT (N = 6) and six Lkb1 KO (N = 6) embryos were analyzed, for each embryo, 60 sections/embryo. Graph shows means ± SEM (unpaired t test NS). (E) Immunoblot analysis of phosphorylation levels of AMPK (Thr172) and acetyl CoA carboxylase (ACC) (Ser72) in soma and axons of DRG explants plated on insert filter cultures for 48 h. (F, G) Phosphorylation levels/total levels of ACC (F) and AMPK (G) were normalized on β-actin and quantified with ImageJ. Graphs show mean ± SEM based on three individual experiments (N = 3). (F, G) ACC: unpaired t test with Welch’s correction soma NS, unpaired t test axons **P = 0.005, (G) AMPK: unpaired t test soma NS, unpaired t test axons *P = 0.04. (H, I) Mitochondrial kymograph of axonal mitochondria motility in dissociated neurons from E13.5 WT and Lkb1 KO DRGs. Scale bar 2 μm. (J) Quantification of the percentage of stationary, anterograde, and retrograde mitochondria in WT and Lkb1 KO neurons. Neurons were dissected from three WT and three Lkb1 KO embryos. All graphs show means ± SEM based on three individual experiments (N = 3), DRGs (stationary mitochondria: unpaired-test NS, anterograde mitochondria: unpaired t test NS, retrograde mitochondria: unpaired t test with Welch’s correction NS).
Source data are available for this figure.
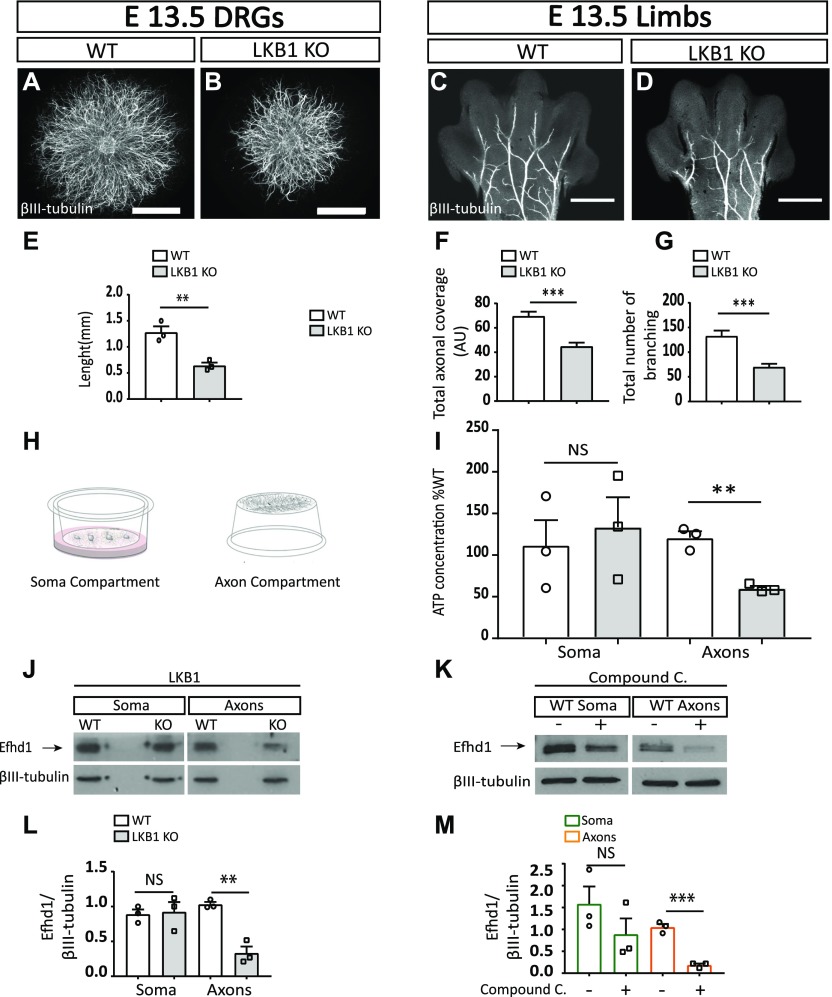
We next examined the importance of Lkb1 for axonal growth in vitro. DRG explants from E13.5 embryos were grown for 5 d embedded in 3D collagen matrix (Fig 1A and B). α-βIII-tubulin staining of the explants revealed that Lkb1 KO axons were significantly shorter (50%) than those of the WT littermate controls (Fig 1E). Thus, unlike hippocampal and cortical neurons (Barnes et al, 2007; Shelly et al, 2007; Courchet et al, 2013), DRG sensory neurons are capable of establishing polarity in the absence of Lkb1, but their axon growth potential is significantly compromised.
Figure 1. Liver kinase B1 (Lkb1) KO embryos display reduced axonal morphogenesis along with metabolic abnormalities and a decrease in the mitochondrial protein Efhd1.
(A, B) Dorsal root ganglion (DRG) explants plated in 3D collagen. Scale bar: 1,000 μm. (C, D) Limbs from WT and Lkb1 KO E13.5 mouse embryos stained with α-βIII-tubulin. Scale bar: 500 μm. (E) Quantification of the axonal length of WT and Lkb1 KO DRGs. Axonal lengths of eight WT and eight Lkb1 KO DRG explants were quantified by four measurements in three independent experiments N = 3. Graphs show means ± SEM (unpaired t test **P = 0.0071). (F) Overall axonal coverage (total axonal length that cover the limb’s surface). Graph shows means ± SEM (unpaired t test ***P = 0.0001). (G) Total number of branching was quantified using NeuroMath. Graph shows means ± SEM (unpaired t test ***P = 0.0001). (F, G) Eight WT (N = 8) and six Lkb1 (N = 6) KO embryos were analyzed in (F, G); data for each embryo represent the average measurements of both limbs. (H) Schematic illustration of the filter cell culture system that facilitates the purification and biochemical analysis of cell bodies and axons. (I) Analysis of ATP levels in WT and Lkb1 KO soma and axons. Graph shows means ± SEM of three independent experiments (N = 3) (unpaired t test: soma NS, unpaired t test: axons **P = 0.0019). Values are presented as the % of WT. (J) Immunoblot analysis of Efhd1 expressions levels in Lkb1 KO and WT soma and axons. (K) Immunoblot analysis of Efhd1 expression in WT DRGs treated with 20 μM compound C for 8 h. (L) Quantification of Efhd1 protein level in Lkb1 KO DRGs compared to WT. Graph shows means ± SEM of three independent experiments (N = 3) (unpaired t test: soma NS, unpaired t test axons **P = 0.00232). (M) Quantification of Efhd1 expression in treated WT DRGs. Graphs show mean ± SEM of three independent experiments (N = 3) (unpaired t test: soma NS, unpaired t test axons ***P = 0.0004).
Source data are available for this figure.
Lkb1 KO sensory neurons exhibit reduced axonal morphogenesis in vivo
Next, we assessed the role of Lkb1 in axonal morphogenesis in vivo. Limbs from E13.5 WT and Lkb1 KO embryos were stained with α-βIII-tubulin and visualized by microscopy (Fig 1C and D). Compared with WT limbs, Lkb1 KO limbs showed reduced axonal morphogenesis. Axonal morphology was quantified using NeuroMath (Rishal et al, 2013). Relative to those in WT mice, limbs in Lkb1 KO mice displayed a strong reduction in the overall axonal coverage of the limb (36%) (Fig 1F) and an even more pronounced decrease in the total number of axonal branches (48%) (Fig 1G). To rule out the possibility that these observed phenotypes are secondary to neuronal death, we counted the number of Islet1 (a pan-sensory neuronal marker)-positive cells in DRGs of E15.5 embryos. We could not detect any significant difference in neuronal numbers between Lkb1 KO and WT control littermates (Fig S1D), suggesting that the axonal abnormalities observed in the Lkb1 KO mice were not caused by cell death. These results highlight the crucial role of Lkb1 as a regulator of developmental axonal morphogenesis in the PNS.
Lkb1 KO neurons display reduced axonal ATP levels
As Lkb1 ablation had no effect on sensory axon polarization, we hypothesized that the reduced axonal growth observed in the KOs in vitro and the reduced axonal morphogenesis phenotypes detected in vivo may be linked to the distinct function of LKB1 in metabolic homeostasis (Alexander & Walker, 2011; Germain et al, 2013; Hawley et al, 2003; Pooya et al, 2014). Because the Lkb1 KO exhibits distinct axonal phenotypes, we determined the ATP concentration in different neuronal compartments using a filter culture system that allows us to differentially examine the cell bodies and the axons (Fig 1H). Whereas there was no difference observed between the ATP levels in Lkb1 KO and WT soma, the ATP level was significantly reduced (49%) in the axons of Lkb1 KO neurons compared with WT (Fig 1I). This dramatic decrease in ATP levels in Lkb1 KO axons was not accompanied by activation of the metabolic sensor AMPK or its direct substrate acetyl CoA carboxylase (ACC), as judged by the extent of their phosphorylation (Fig S1E–G). This suggests that Lkb1 is a nonredundant activator of AMPK in sensory neurons. Taken together, these in vitro and in vivo results demonstrate that Lkb1 serves to maintain normal axonal development and ATP levels in mouse PNS neurons.
Lkb1-deficient sensory axons display normal mitochondria motility
As mitochondria are the main source of axonal ATP and Lkb1 controls the axonal movement of the mitochondria in cortical neurons (Courchet et al, 2013), we tested the motility of mitochondria in sensory axons of DRG neurons from Lkb1 KO and WT mice. Lkb1 KO and WT neurons were plated on poly-D-lysine (PDL)/laminin–coated microfluidics chambers for 72 h (Maor-nof et al, 2016). The mitochondria were labeled by TMRE, which localizes to mitochondria, and the cells were imaged. No significant difference in mitochondrial motility was detected (Fig S1H–J).
Lkb1 KO sensory neurons exhibit reduced expression of the mitochondrial protein Efhd1
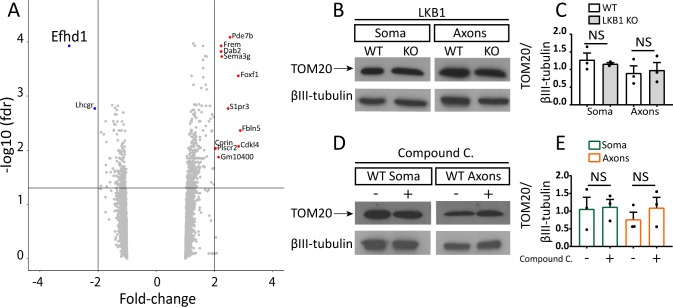
In addition to directly phosphorylating its downstream targets, Lkb1 signals activation of gene transcription (Shackelford & Shaw, 2009). Therefore, to identify differential gene expression in DRGs directly isolated from E13.5 WT and Lkb1 KO embryos, we performed transcriptome profiling using microarray analysis. Surprisingly, there were very few alterations in gene expression in Lkb1 KO DRGs compared with WT controls. Most profound was the reduction in the expression of Efhd1, a mitochondrial Ca2+-binding protein (approximately threefold) (Fig S2A and Table S1). In support, we detected a significant decrease in axonal Efhd1 protein expression in Lkb1 KO neurons compared with WT (Fig 1J and L). To further test the connection between Efhd1 expression and the Lkb1 pathway, we treated DRG neurons in vitro with the AMPK inhibitor compound C. After 8 h, we detected a profound reduction in axonal Efhd1 (Fig 1K and M). We have not detected a reduction in the levels of the mitochondrial protein TOM20 in the Lkb1 KO or compound C–treated WT neurons (Fig S2B–E), suggesting that the reduction in Efhd1 is not due to global reduction in mitochondrial proteins.
Figure S2. Efhd1 is down-regulated in Liver kinase B1 (Lkb1) KO neurons.
(A) Volcano plot of microarray analysis of dorsal root ganglions (DRGs) directly isolated from E13.5 embryos. (B) Immunoblot analysis of TOM20 expression using the filter cell culture system in WT and Lkb1 KO E13.5 DRGs after 48 h of growth. (C) Quantification of TOM20 protein level to tubulin ratio in WT and Lkb1 KO DRGs (unpaired t test soma and axons NS). (D) Immunoblot analysis of TOM20 expression using the filter cell culture system in WT DRGs treated with compound C for 8 h after 48 h of growth. (E) Quantification of TOM20 protein level to tubulin ratio in WT DRGs treated with compound C (unpaired t test soma and axons NS). All graphs show means ± SEM based on three individual experiments (N = 3).
Source data are available for this figure.
Table S1 Gene expression analysis of WT and Lkb1 KO E13.5 DRGs.Gs. (5.2MB, xls)
Overall, these data show that Lkb1 pathway inhibition causes Efhd1 down-regulation in DRG neurons.
Efhd1 is required for axonal growth in vitro
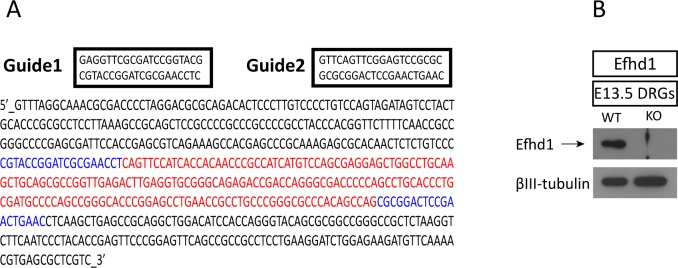
To investigate the physiological function of Efhd1, we generated an Efhd1 KO mouse using the CRISPR–Cas9 technology (Fig S3A). Complete elimination of Efhd1 expression was confirmed by Western blot (Fig S3B). To determine if Efhd1 is required for axonal growth in DRG neurons in vitro, we first cultured DRG explants from WT and Efhd1 KO E13.5 embryos for 48 h on PDL/laminin, which is highly permissive. Under these culture conditions, we have not observed any effect on axonal growth (Fig S4A–C). Next, we cultured E13.5 DRGs for 5 d in 3D collagen matrix. Axons were visualized by α-βIII-tubulin staining (Fig 2A and B). Under these conditions, axons of the Efhd1 KO neurons measured consistently shorter (18%) compared with those from WT littermates (Fig 2E). These results are reminiscent of the Lkb1 KO phenotype and are consistent with previous studies on Efhd1 (Tominaga et al, 2006), supporting the notion that Efhd1 is required for axonal growth in vitro.
Figure S3. Generation of the Efhd1 KO.
(A) Schematic of Efhd1 KO strategy by CRISPR. The appropriate section of DNA (marked in red, including the promoter and first exon) was deleted using two guides (marked in blue). (B) Immunoblot analysis of Efhd1 protein expression in WT and Efhd1 KO DRGs directly isolated from E13.5 embryos.
Source data are available for this figure.
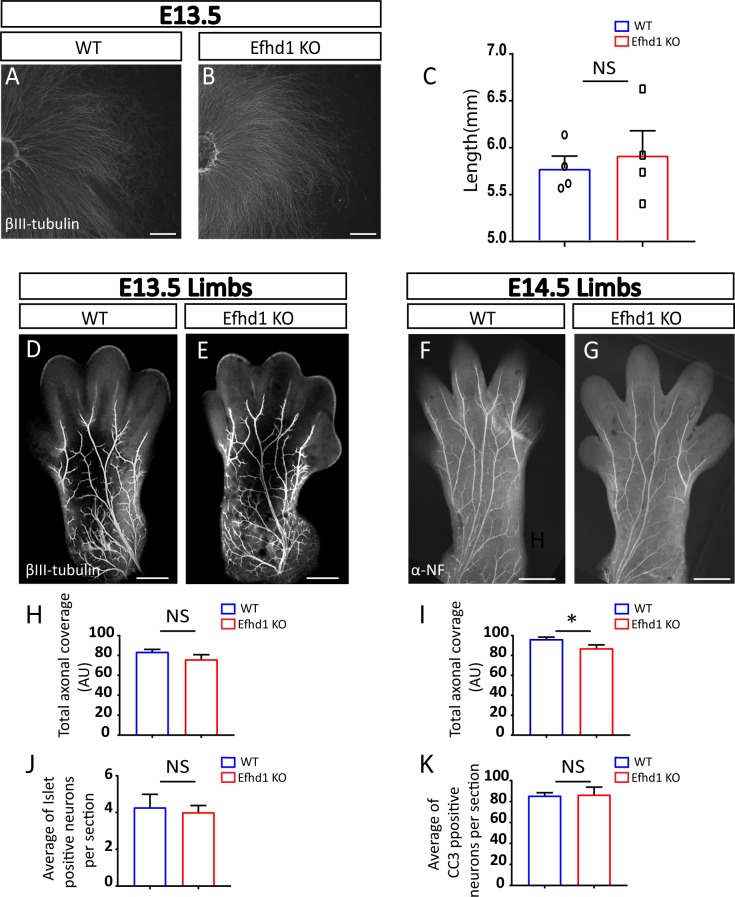
Figure S4. Efhd1 KO embryos show reduced axonal growth in vivo.
(A, B) Dorsal root ganglions (DRGs) from E13.5 embryos from WT and Lkb1 KO E13.5 mouse embryos were plated on poly-D-lysine/laminin and stained with α-βIII-tubulin. Scale bar 1,000 μm. (C) Axonal length of eight WT and eight Efhd1 KO DRG explants were quantified by four measurements in four independent experiments (N = 4). Graphs show means ± SEM (unpaired t test NS). (D, E) α-βIII-tubulin immunostaining of E13.5 limbs of WT and Efhd1 KO embryos. Scale bar 500 μm. (F, G) Analysis of axonal morphogenesis in E14.5 limbs of WT and Efdh1 KO embryos as visualized by α-neurofilament staining. Scale bar 500 μm. (H) Quantification of overall axonal coverage (total axonal length that cover the limb’s surface) in WT and Efhd1 KO E13.5 limbs. Five WT embryos (N = 5) and nine Efhd1 KO (N = 9) embryos were analyzed; data for each embryo represent the average axonal coverage of both limbs. Graphs show mean ± SEM (unpaired t test NS). (I) Quantification of the overall axonal coverage (total axonal length that cover the limb’s surface) in WT and Efdh1 KO E14.5 limbs. Six WT embryos (N = 6) and eight Efhd1 KO (N = 8) embryos were analyzed; data for each embryo represent the average of axonal coverage of both limbs. Graphs show means ± SEM (unpaired t test *P = 0.04). (J, K) Quantification of (J) Islet1 neurons and (K) apoptotic rate (anti-cleaved caspase-3 staining [CC3] in E15 WT and Efhd1 KO DRGs). Six embryos of each genotype were analyzed (N = 6), with 60 sections/embryo. Graphs show mean ± SEM (unpaired t test NS).
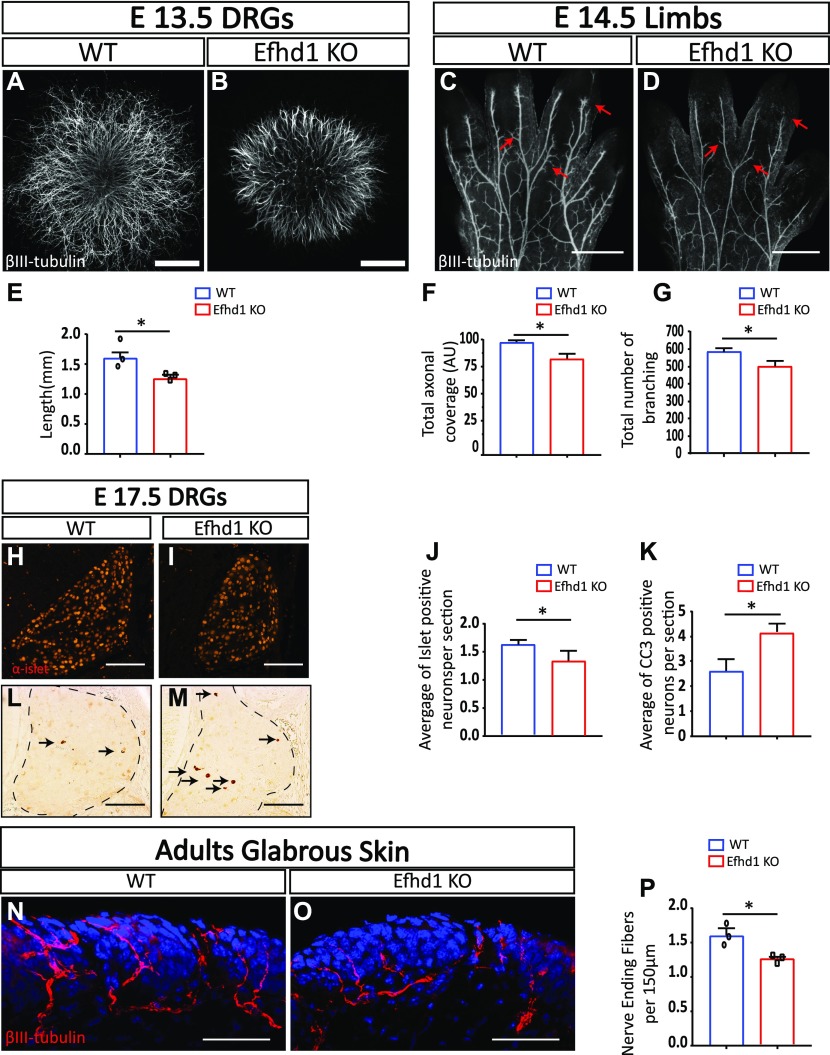
Figure 2. Efhd1 KO neurons exhibit reduced axonal morphogenesis, neuronal cell loss, and decreased target innervation.
(A, B) Dorsal root ganglion (DRG) explants placed in 3D collagen from WT and Efhd1 KO E13.5 mouse embryos stained with α-βIII-tubulin. Scale bar: 1,000 μm. (C, D) Limbs from WT and Efhd1 KO E14.5 mouse embryos, stained with α-βIII-tubulin. Arrows point missing axonal branches in the Efhd1 KO. Scale bar: 500 μm. (E) Axonal lengths of eight WT and eight Efhd1 KO DRGs were quantified by four measurements in three independent experiments (N = 3). Graph shows means ± SEM (unpaired t test *P = 0.0237). (F) Overall axonal coverage (total axonal length that cover the limb’s surface). Graph shows means ± SEM (unpaired t test *P = 0.038). (G) Total number of branching was quantified. Graphs show means ± SEM (unpaired t test *P = 0.032). (F, G) Seven WT (N = 7) and eight Efhd1 KO (N = 8) embryos were analyzed in (F, G), data for each embryo represent the average measurements of both limbs. (H, I) E17.5 WT and Efdh1 KO mouse embryos were stained with anti-Islet1. Scale bar: 100 μm. (J) Graph shows means ± SEM (unpaired t test *P = 0.011). (L, M) E17.5 WT and Efdh1 KO mouse embryos were stained with anti-cleaved caspase-3 (CC3). Scale bar: 100 μm. (K) Graph shows means ± SEM (unpaired t test *P = 0.020). Arrows point to the CC3-positive cells in the DRGs. (J, K) Five WT (N = 5) and seven Efhd1 KO (N = 7) embryos were analyzed, and the numbers of Islet1-positive neurons (J) and of CC3 positive (K) were quantified in 60 sections/embryo. (N, O) Glabrous skin of adult hind limbs from WT and Efdh1 KO adult mice was stained with α-βIII-tubulin and visualized by confocal microscopy. Scale bar 100 μm. (P) Nerve-ending fiber quantification. Graph shows means ± SEM (unpaired t test *P = 0.013). 25 sections from each left hind limb of three WT (N = 3) and three Efhd1 KO (N = 3) adult animals were analyzed.
Efhd1 KO sensory neurons display aberrant axonal development and increased cell death in vivo
Next, we assessed whether Efhd1 is also required for axonal morphology in vivo. To this aim, we visualized Efhd1 KO and littermate WT limbs of E13.5 and E14.5 embryos and quantified axonal morphology by NeuroMath. At E13.5, the axonal patterns in WT and KO appeared identical (Fig S4D, E, and H). However, at E14.5 (Fig 2C and D), we detected a significant 18% reduction in the overall axonal coverage (Fig 2F) and a 19% decrease in the total number of branches (Fig 2G). Similar results were obtained using α-neurofilament staining (Fig S4F, G, and I).
We then investigated whether the lack of Efhd1 provoked neuronal loss. To assess the extent of cell death in our system, we stained for the pan-DRG neuronal marker, Islet1, and processed (i.e., active) caspase-3, which is an indicator for apoptotic cell death. No differences were noted at E15.5 between the numbers of neurons and the apoptotic rates in Efhd1 WT and KO DRGs (Fig S4J and K). In contrast, at E17.5, a significant decrease in neuronal numbers (28%) was detected in Efhd1 KO DRGs (Fig 2H–J), along with an increased rate of apoptotic cells (36%) (Fig 2L, M, and K).
Having demonstrated that the aberrant phenotypes in Efhd1 KO mice begin to arise during early development, we examined whether innervation in adult animals is also affected. After staining the hind limb glabrous skin with α-βIII-tubulin antibody (Fig 2N and O), we detected a significant reduction (33%) in the number of terminal fibers in Efhd1 KO hind limb skin compared with the WT control (Fig 2P). Overall, these data suggest that Efhd1 is required in sensory neurons for axonal morphogenesis during development and for proper target innervation.
Efhd1 KO axons manifest lower ATP levels and shortened mitochondria
As Efhd1 is a mitochondrial protein, we examined whether its ablation affected energy homeostasis of the neuron. We measured ATP levels in WT and Efhd1 KO neurons from E13.5 embryos and found that whereas no significant differences in ATP levels were observed between the corresponding WT and KO DRGs somas, the ATP levels of Efdh1 KO axons are much lower (49%) than that of WT axons (Fig 3A).
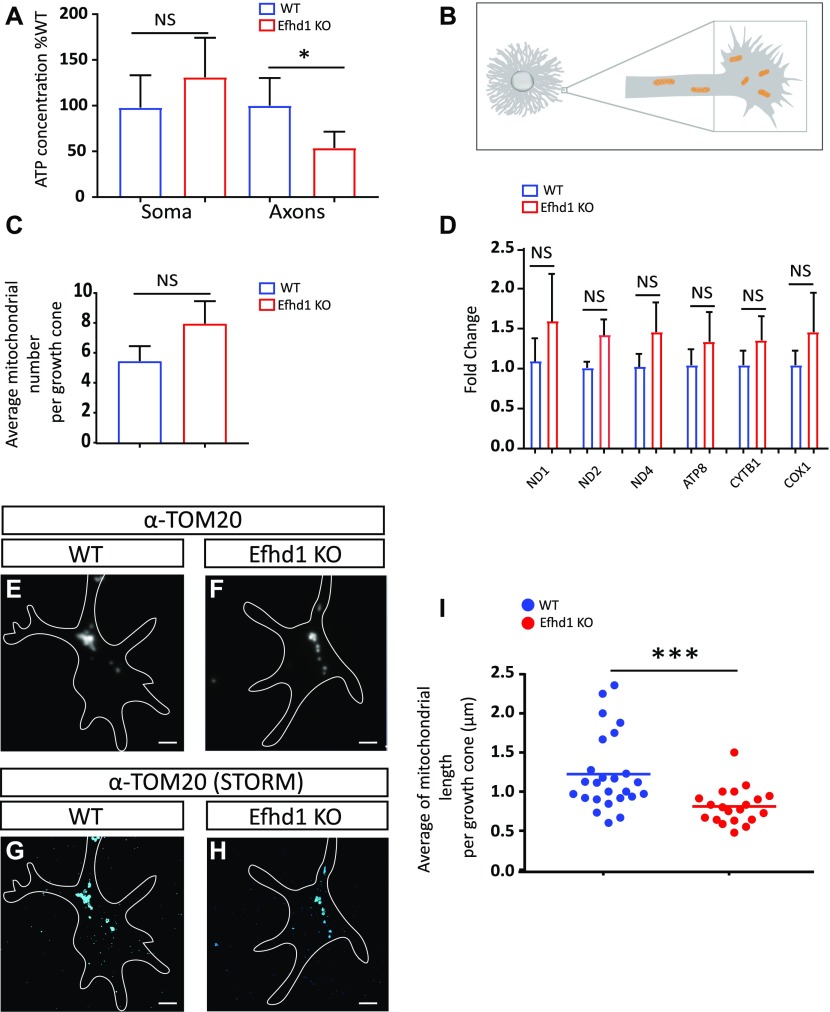
Figure 3. Efhd1 KO axons exhibit decreased ATP levels and shortened mitochondria.
(A) Soma and axonal ATP levels of cultured dorsal root ganglion neurons were measured using the filter cell culture system. Values are presented as the % of WT. Graph shows means ± SEM of 12 independent experiments (N = 12) (Wilcoxon signed rank test: soma = NS, axons *P = 0.0034). (B) Schematic illustration of the plated dorsal root ganglion explant, axon and growth cone (marked by square) from which mitochondria morphology and number were analyzed by TOM20 staining. (C) Quantification of the number of mitochondria per growth cones counted in the super-resolution images. Graphs show means ± SEM of 25 growth cones WT and 20 Efhd1 KO (unpaired t test NS). (D) Real-time PCR analysis of six mitochondrial genes (ND1, NADH dehydrogenase subunit 1; ND2, NADH dehydrogenase subunit 2; ND4, NADH dehydrogenase subunit 4; ATP8, ATP synthase protein 8; CYTB1, cytochrome b COX1: cytochrome c oxidase subunit 1). Graph shows means ± SEM of four independent experiments (N = 4) (unpaired t test all NS). (E, F) Wide-field fluorescence image of anti-TOM20 staining. Scale bar 2 μm. (E, F, G, H) Super-resolution (STORM) images of anti-TOM20 staining of the same area of (E, F). Scale bar 2 μm. (I) Quantification of mitochondria length: each point represents the average mitochondria length in a single growth cone; >150 mitochondria were analyzed from each genotype, N = 25 WT growth cones and N = 20 Efhd1 KO growth cones. Graph shows means ± SEM (Mann–Whitney test: ***P = 0.0002).
Having established that axonal ATP is impacted by Efhd1 deficiency, we reasoned that mitochondria numbers or morphology might also be affected. We examined mitochondria number in Efhd1 KOs using two approaches. First, we directly counted the number of mitochondria at the axonal growth cones of DRG explant (see schematic illustration in Fig 3B) after visualizing them using super-resolution microscopy. No significant difference in mitochondrial number was observed between the WT and Efnd1 KO DRGs (Fig 3C). Next, we quantified the total mitochondria mass in DRG neurons by conducting real-time PCR analysis of six mitochondrial genes (Maryanovich et al, 2015; Ruggiero et al, 2017). No significant differences were detected between WT and Efnd1 KO DRGs by this approach as well (Fig 3D).
In parallel, we examined mitochondria morphology of the above samples. The mitochondria in Efhd1 KO growth cones were significantly shortened compared with those in WT organelles (0.77 versus 1.13 μm, respectively) (Fig 3E–I). Together, these results demonstrate that lack of Efhd1 results in metabolic dysfunctions in sensory axons, which correlates with reduced axonal morphogenesis and aberrant mitochondria morphology.
Ablation of Efhd1 causes mitochondrial dysfunction in sensory neurons
Our observations that Efhd1 KO neurons have similar number of axonal mitochondria with irregular morphology prompted us to directly examine the mitochondrial oxidative phosphorylation (OXPHOS)-mediated ATP production activity in these cells. We used the oxygen consumption rate, as measured by the Seahorse XF96 system, as a readout of OXPHOS. An equal number of E13.5-dissociated DRGs were plated on PDL/laminin for 4 d, and the mitochondrial activity measurements were preformed according to Styr et al (2019). The Efhd1 KO neurons exhibited clear reduction in the basal mitochondria respiration (Fig 4A–C), ATP-linked respiration, and ATP production (Fig 4A and D). We also detected significant reduction in the spare respiratory capacity (SRC) based on multi-comparison Sidak’s test (Fig 4B). However, when the SRC was calculated according to the baseline of each genotype, we did not detected any significant difference (Fig 4E). Therefore, we cannot conclude that the SRC is defective in the Efhd1 KO. No differences were detected in the non-mitochondrial respiration (proton leak) activity (Fig 4A, B, and F). Overall, our data show that Efhd1 is required for mitochondrial activity and ATP production under basal conditions in sensory neurons.
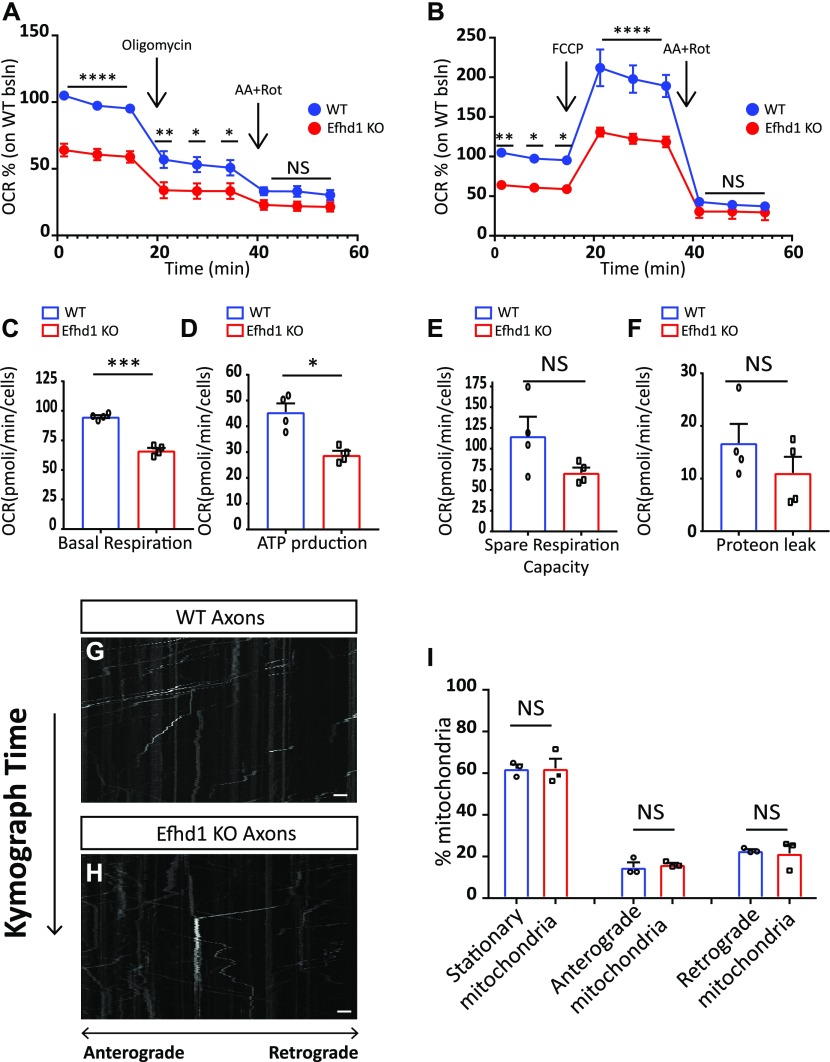
Figure 4. Efhd1 KO neurons show decrease in mitochondrial activity.
(A, B) Analysis of the oxygen consumption rates (OCRs) in dissociated dorsal root ganglion (DRG) from E13.5 WT and Efhd1 KO embryos by the Seahorse Bioscience XF96 analyzer. (A, B) First, the basal respiration was measured, then (A) oligomycin (1 μM) was added to measure the ATP production, and (B) FCCP (4 μM) was added to measure the spare respiratory capacity. The respiration was stopped and non-mitochondrial oxygen consumption was measured after injection of 0.5 μM rotenone (Rot) and 0.5 μM antimycin A (AA). Data are presented as the % of the WT baseline (bsln). The analysis is based on four independent biological experiments, (N = 4) in each experiment, at least nine wells were used for each condition for each genotype. (A, B) Two-way ANOVA test was performed with Sidak’s multi-comparison test: (A) Baseline point 1-2-3,****P ≤ 0.0001, Oligomycin point 1 **P = 0.004, point 2 *P = 0.0195 point 3 *P = 0.0462, rotenone and antimycin P = NS; (B) baseline point 1 **P = 0.0041, point 2 *P = 0.0132, point 3 *P = 0.0147, FCCP point 1-2-3 ****P ≤ 0.0001, rotenone and antimycin P = NS. (C, D, E, F) Graphical quantification of (C) basal respiration, (D) ATP production, (E) spare respiratory capacity, and (F) non-mitochondrial ATP production (proton leak) (C: unpaired t test ***P = 0.001, D: unpaired t test **P = 0.0042, E, F: unpaired t test NS). Graphs show means ± SEM of OCR that are based on four independent biological experiments (N = 4), in each experiment, at least nine wells were used for each condition for each genotype. (G, H) Mitochondrial kymograph of axonal mitochondria motility in dissociated neurons from E13.5 DRGs WT and Efhd1 KO. Scale bar 2 μm. (I) Quantification of the percentage of stationary, anterograde, and retrograde mitochondria in WT and Efhd1 KO neurons. All graphs show mean ± SEM based on three experiments (N = 3); DRGs were dissected from three WT (N = 3) and three Efhd1 KO (N = 3) embryos (stationary mitochondria: unpaired test NS, anterograde mitochondria: unpaired t test NS, retrograde mitochondria: unpaired t test with Welch’s correction NS).
Last, we tested if the deficits in mitochondrial activity are also associated with changes in mitochondrial motility. Efhd1 KO and WT neurons were plated on PDL/laminin-coated microfluidics chambers for 72 h (Maor-nof et al, 2016). The mitochondria were labeled by MitoTracker, which localizes to mitochondria regardless of their membrane potential, and the cells were imaged for 6 h (Ionescu et al, 2016). We did not detected any change in the overall pattern of mitochondrial motility in the Efhd1 KO neurons (Fig 4G–I).
Efhd1 KO sensory neurons have activated metabolic stress signaling
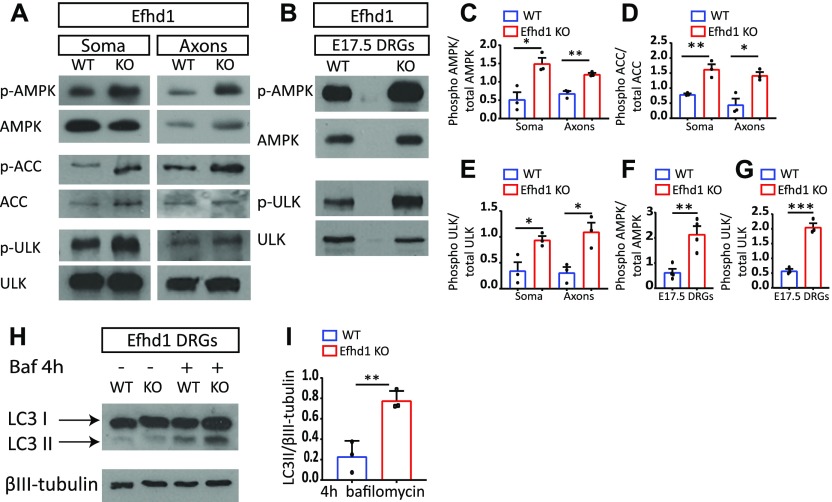
The mitochondrial dysfunction observed in Efdh1 KO axons prompted us to examine the activation status of AMPK in those neurons. We discovered that, unlike in Lkb1 KOs, AMPK is hyper-phosphorylated in Efhd1 KO axons and DRGs as compared with the WT control, both in vitro (Figs 5A and C and S5A) and in vivo (Figs 5B and F and S5B). Consistently, we also detected higher phosphorylation of the canonical AMPK target, ACC, in the soma and axons of Efhd1 KO DRGs compared with WT (Figs 5A and D and S5C). As AMPK also regulates the recycling of aberrant mitochondria via mitophagy through stimulation of Ulk (Toyama et al, 2016; Zhang & Lin, 2016), we tested whether this pathway is enhanced in Efhd1 KOs. In line with AMPK activation, Ulk serine-555 phosphorylation was markedly elevated in the Efhd1 KO DRGs both in vitro (Figs 5A and E and S5D) and in vivo (Figs 5B and G and S5E).
Figure 5. Efhd1 KO sensory neurons display AMPK and Ulk activation along with increased autophagic flux.
(A) Immunoblot analysis of AMPK and Ulk activation in E13.5. Dorsal root ganglion (DRG) explants were grown on the filter cell culture system for 48 h. AMPK (Thr172), ACC (Ser79), and Ulk (Ser555) phosphorylation status were analyzed. (B) AMPK (Thr172) and Ulk (Ser55) phosphorylation status were analyzed by immunoblot of in vivo (directly extracted) E17.5 DRGs. (C, D, E, F, G) The ratio of phosphorylated to total levels of protein normalized to tubulin was quantified using ImageJ levels in E13.5 (C, D, E) and E17.5 (F, G) DRGs was quantified using ImageJ. (C, D, E) AMPK: soma *P = 0.0168, axons **P = 0.0022, (D) ACC: soma **P = 0.0069, axons *P = 0.0131, (E) Ulk: t test soma *P = 0.0287, axons *P = 0.0185. Graphs show means ± SEM based on three independent experiments (N = 3), unpaired t test was used for all. (F, G) AMPK: t test **P = 0.0038 (G) t test ***P = 0.0005. All graphs show means ± SEM based on N = 4 (p-AMPK) and N = 3 (p-Ulk) independent experiments, unpaired t test was used for both. (H) Immunoblot of LC3I and LC3II levels of WT and Efhd1 KO DRGs treated with bafilomycin (100 nM) for 4 h. (I) The ratio of LC3-II to tubulin was quantified with ImageJ. Graphs show means ± SEM based on three independent experiments (N = 3) (unpaired t test **P = 0.006).
Source data are available for this figure.
Figure S5. Immunoblot analysis presented with relative loading controls.
(A, B, C, D, E) Complete immunoblot analysis with loading controls that were used for the quantifications in Fig 5.
Next, we tested if there is an increase in autophagic flux in Efhd1 KO neurons. We cultured WT and Efhd1 KO DRG neurons in the presence of the lysosomal inhibitor bafilomycin. Under these conditions, autophagic vesicles are not degraded, allowing accumulation of the lipidated form of the LC3 protein (Zhang et al, 2012; Jiang & Mizushima, 2015; Redmann et al, 2017). Although, as expected, bafilomycin caused an increase in the levels of lipidated LC3 (LC3-II) in WT neurons, we detected a further increase in LC3-II levels in the Efhd1 KO neurons (Fig 5H and I). These results support the notion that autophagic flux is enhanced in Efhd1 KO cells, presumably to recruit and recycle the aberrant, dysfunctional mitochondria. This agrees with our finding that AMPK and Ulk are activated in Efhd1 KO cells. Overall, these results suggest that Efhd1 ablation triggers the activation of metabolic stress pathways in an attempt to compensate for metabolic deficits.
Discussion
In this study, we have identified a metabolic regulator of axonal morphogenesis. We generated and characterized a mouse model that lacks the key metabolic regulator Lkb1. Our data demonstrate that when Lkb1 is ablated in the PNS during early development, neurons display reduced axonal growth both in vivo and in vitro. Cultured Lkb1 KO neurons exhibit a decrease in axonal ATP levels (Fig 6A). Despite this decrease and the consequently elevated cellular stress level, we did not detect increased phosphorylation of AMPK, which is considered to be the sensor of cellular stress (Hawley et al, 2003; Hardie, 2007). These data are in line with the abundance of reports demonstrating that Lkb1 is the principal AMPK kinase (Hawley et al, 2003; Shackelford & Shaw, 2009; Alexander & Walker, 2011).
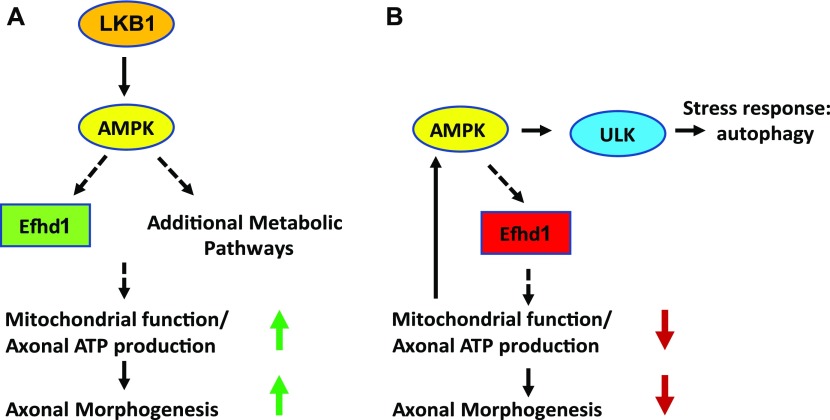
Figure 6. Regulation of axonal morphogenesis by Efhd1.
(A) Lkb1 controls mitochondrial homeostasis and axonal ATP production, through Efhd1 and additional pathways that are required for axonal morphogenesis. (B) Complete ablation of Efhd1 causes reduction in the axonal ATP levels and mitochondrial abnormalities. This results in the activation of AMPK and Ulk and increased autophagic flux, which is correlated with reduced axonal morphogenesis.
To identify new regulators of energy homeostasis, we searched for alterations of gene expression in Lkb1 KO cells. The expression of Efhd1 was significantly reduced in the KO neurons. We also found it to be down-regulated in sensory neurons upon pharmacological inhibition of AMPK. These results imply that Efhd1 might be an effector of the Lkb1–AMPK pathway. Additional studies are required to establish if the Lkb1–AMPK pathway directly controls Efhd1 levels, and if so, the exact mode of regulation.
Our results suggest that Efhd1 is required in sensory neurons for mitochondrial morphology and function. Interestingly, effects of its ablation are mostly manifested in the axons. This may be due to the fact that axons are more sensitive to mitochondrial dysfunction because of their length or because other metabolic pathways compensate more efficiently in the soma. Our in vivo analysis also supports the idea that axons are more sensitive to the lack of Efhd1, as we detected axonal phenotypic changes as early as E14.5, whereas enhanced cell death was only observed at E17.5.
Our biochemical analysis shows that the Efhd1 KO neurons respond to mitochondrial dysfunction by activating metabolic stress pathways, as manifested by the hyper-phosphorylation of AMPK. Although activated AMPK normally rescues the metabolic cellular function in stress condition (Gwinn et al, 2008; Toyama et al, 2016), in late stages of DRGs development (E17.5), we observed an increased incidence of apoptotic death of Efhd1 KO cells, suggesting that the energy imbalance at this stage cannot be completely resolved despite enhanced AMPK activity. Consistent with the higher apoptotic rate observed at E17.5, innervation of the adults Efhd1 KO skin was significantly sparser compared with WT.
The presence of aberrantly dysfunctional mitochondria in the Efhd1 KO axons raises the notion that mitophagy might be activated in Efhd1 KO DRGs. Indeed, we found enhanced Ulk phosphorylation at serine-555, which facilitates mitophagy in response to AMPK activation and increases the overall autophagic flux (Toyama et al, 2016) (Fig 6B). In line with our results, a recent study revealed that mitochondria shortening and the fission process can be directed by AMPK through the mitochondrial fission factor protein. Once AMPK is activated in response to cellular stress, the mitochondrial fission factor is phosphorylated and promotes the constriction and fission of mitochondria, prompting their engulfment by mitophagosomes (Toyama et al, 2016; Zhang & Lin, 2016).
The exact mitochondrial function of Efhd1 remains to be discovered. Previous studies linked Efhd1 to metabolic regulation in the development of bone marrow cells (Stein et al, 2017) and to the activation of mitoflashes, short stochastic superoxide bursts during cellular respiration (Hou et al, 2016). Notably, Efhd1 modulation was described earlier in a neuronal cell line where Efhd1 overexpression resulted in neurite extension, whereas its down-regulation caused neurite reduction and subsequent cell death (Tominaga et al, 2006). Given its calcium-binding capacity (Tominaga et al, 2006), it is tempting to speculate that Efhd1 may serve as a new link between the mitochondrial Ca2+ and the mitochondria OXPHOS. Multiple studies have clearly demonstrated that Ca2+ is a physiological regulator of OXPHOS and that it can facilitate the activation of several enzymes in the tricarboxylic acid (TCA) cycle (Rizzuto et al, 2012). Upon Ca2+ binding by its EF-hand domain, Efhd1 may directly interact with proteins of the OXPHOS machinery in the mitochondrial inner membrane and stimulate their activity. Then, when Efhd1 function is impaired, either partially or completely (as in Lkb1 KO and Efhd1 KO, respectively), this Ca2+-dependent regulation of ATP production would be compromised. This may trigger mitochondrial stress due to unmet energy demand during axonal growth, provoking mitochondria recycling through mitophagy.
Despite their sensory neurons’ mitochondrial dysfunction, resultant aberrant axonal growth, and neuronal loss, mice with Efhd1 deficiency are viable, are fertile, and have normal appearance and behavior in the cage. Moreover, Efhd1 KO DRGs and axons display only limited defects in vitro and in vivo, highlighting the specific contribution of Efhd1 to axon-remodeling processes but not to basal homeostasis of the soma.
This study presents a new pathway of mitochondrial regulation. Lkb1, already known as a master metabolic regulator (Shaw et al, 2004; Shackelford & Shaw, 2009; Alexander & Walker, 2011; Pooya et al, 2014) modulates expression of the mitochondrial protein Efhd1. As demonstrated herein, Efhd1 has a unique and crucial role in axonal development of the PNS.
Materials and Methods
Mouse strains: Lkb1 and Efhd1
The Lkb1 conditional KO mouse line was established by crossing Lkb1 flox mice (Swisa et al, 2015) with Wnt1–Cre transgenic mice. The Wnt1–Cre transgene is expressed in neural crest cells (progenitors of [DRG] neurons and glia), and in the midbrain and dorsal neural tube of the CNS (Charron et al, 2003). Wnt1 expression starts at the embryonic day 8 (E8) in the midbrain and is expressed in its full pattern by E9 (Charron et al, 2003). The Lkb1 KO mice die shortly after birth.
The Efhd1 KO mouse line was generated by CRISPR–cas9 technique. The following two guide RNAs were used to delete a part of the Efhd1 gene coding region and its promoter:
Guide 1: sgRNA1-top: CACCgGAGGTTCGCGATCCGGTACG, sgRNA1-bottom: AAACCGTACCGGATCGCGAACCTCc.
Guide 2: sgRNA2-top: CACCgGTTCAGTTCGGAGTCCGCGC, sgRNA2-bottom: AAACGCGCGGACTCCGAACTGAACc.
The Efhd1 KO mice are viable and appear indistinguishable from their WT sibs in cage environment. Mice are of mix genetic background in all of the experiments, and we used littermate (WT) controls. Mice were hosed in specific pathogen-free facility, under 50% humidity, 12-h light/dark cycle, 22c with standard diet (18% protein, 5% fat). All animal experiments followed protocols approved by The Weizmann Institute of Science Institutional Animal Care and Use Committee.
Explant culture, medium, and culture method
DRG explants of E13.5 mice were aseptically removed from E13.5 embryos in L15 medium (L15 powder [MFCD00217482; Sigma-Aldrich]) dissolved in filtered distilled water (FDW) with 5% fetal bovine serum (10091148; Gibco). Chambers and filter plates: insert (cell culture insert, six-well hanging insert, 1 μm PET, MCRP06H48; Millicell), eight chambers (cell culture slide eight well, 30108; Life Science Co. Ltd.), six-well plate (140675; Thermo Fisher Scientific), and cell culture dish (430156; Merck). The coating was done for 1 h with PDL (P6407; Sigma-Aldrich) diluted in FDW, final concentration 0.01 mg/ml and, after washing briefly with FDW, laminin (114956-81-9; Sigma-Aldrich), and diluted in filtered F12 (01-095-1A; Biological Industries) for 2 h at 37°C at a final concentration of 10 μg/ml. The plated explants were grown for 48 h in complete NB (10888022; Thermo Fisher Scientific) supplemented with 2% B27 (17054-044; Thermo Fisher Scientific), 1% penicillin–streptomycin solution (03–0311B; Biological Industries), 1% glutamine (25030081; Gibco), and 12.5 ng/ml NGF (CAS 866405-64-3).
For protein analysis, DRGs were plated in the insert, growth for 48 h in NB plus NGF, and the cell bodies and the axons were lysed followed by Western blot of the samples. When indicated, 20 μM compound C (CAS 866405-64-3; Sigma-Aldrich) for 8 h or 100 nM bafilomycin (LC Laboratories) for 4 h was added after 48 h of cell growth in NB. The cultures were then lysed and processed for Western blot.
For immunofluorescent staining, DRGs were plated in eight chambers or cell culture plates for 48 h in NB plus NGF, fixed with 4% formaldehyde for 1 h at room temperature, and stained using mouse tubulin βIII antibody. Images were taken with DS-Qi2 fluorescent microscope, Nikon.
3D collagen cultures
Collagen cultures were performed as previously described (Charron et al, 2003; Romi et al, 2014). Briefly, DRGs, dissected as described above, were embedded in 2 mg/ml collagen matrix (1179179001; Roche) supplemented with NB plus NGF. After 5 d in culture, the explants were fixed with 4% formaldehyde and stained using mouse tubulin βIII antibody. DRGs were visualized with Leica MZ16F binoculars (Nikon), axonal length was measured using ImageJ software.
Sample lysis and immunoblot quantification
Two different cell lysis buffers were used for Western blot analysis:
-
1)
RIPA buffer (50 mM Tris, pH 7.4, 150 mM NaCl, 1% NP40, 0.1% SDS, 0.5% deoxycholate, and 1 mM EDTA in double distilled water) supplemented with cOmplete protease inhibitor cocktail (5892791001; Roche), PMSF (CAS 329-98-6; Sigma-Aldrich), and phosphatase inhibitor cocktails 1 and 2 (P5726; Sigma-Aldrich).
-
2)
Triton-lysis buffer (150 mM NaCl, 10% glycerol, 1% Triton X-100, and 10 mM Tris, pH 7.4, in double distilled water) supplemented with cOmplete protease inhibitor cocktail, PMSF, and phosphatase inhibitor cocktails 1 and 2.
All the samples were lysed in RIPA buffer (1), except the samples from E13.5 embryos used to detect P-Ulk ser 555/Ulk tot, P-ACC/ACC tot, for which the lysis was performed with lysis buffer (2). Immunoblots for P-AMPK, P-Ulk, and LC3 I-II at E13.5 and P-Ulk and P-AMPK at E17.5 were loading-normalized by re-probing the original membranes with the respective loading control (tubulin βIII or β-actin) and total protein antibodies (total AMPK, Ulk, and tubulin βIII for LC3). The immunoblots were scanned and analyzed using ImageJ program. The samples for assessment of P-ACC/ACC at E13.5 were split and loaded on two pairs of identical gels. The intensities of the phosphorylated and the total forms detected on the first set of gels were then normalized on the tubulin βIII or β-actin as detected on the corresponding replica gel set, and then the values of the phosphorylated form/tubulin βIII or phosphorylated form/β-actin ratios were further adjusted relative to the ratios of total/tubulinβIII or total/β-actin.
Immunostaining
After fixation with 4% formaldehyde. DRGs placed in the collagen matrix were gently washed with PBS. Blocking was done with 3% BSA (160069; MP Biomedicals, LLC) and 0.1% Triton X-100 (CAS900-93-1; Sigma-Aldrich) in PBS for 1 h. The blocking solution was used for incubation with primary and secondary antibodies. PBS washes were made between each step.
Whole-mount embryo limbs
Limbs of E13.5 and E14.5 embryos were stained with anti-tubulin βIII antibody and anti-neurofilament antibody 2H3 following the iDisco methodology (Renier et al, 2014). For total axonal coverage and branching quantification, the NeuroMath software was used (Rishal et al, 2013). The numbers of samples processed in each experiment are specified in the corresponding figure legends.
Gene expression microarray
DRGs from E13.5 Lkb1 KO and WT embryos were harvested and RNA extracted as described in Rio et al (2010). For each sample, we pooled DRGs from two embryos. Overall, we analyzed the data from 10 microarrays, profiling five Lkb1 KO and five WT samples. cDNA library construction and microarray hybridization were performed at the microarray unit of the Weizmann Institute of Science. Data were analyzed using MATLAB, false discovery rate 0.01.
Data are available at https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE146756.
Immunohistochemistry of mouse embryos sections
E15.5–E17.5 embryos were fixed for 24 h in 4% formaldehyde and stained as described in Maor-nof et al (2016) with anti-Islet and anti-cleaved caspase-3 antibody. Numbers of embryos and sections used in individual experiments are specified in the figure legends.
The number of Iset1-positive neurons per DRG was calculated by computational approach as described in detail in Maor-nof et al (2016). The number of cleaved caspase-3–positive neurons was manually counted in ImageJ.
Adult skin innervation
The hind limb glabrous skin of 2-mo-old mice was stained as described in Marvaldi et al (2015) (Zylka et al, 2005). For the nerve quantification, the fiber number per 150 μm in the selected epidermis area was counted as described in Marvaldi et al (2015). The numbers of mice, limbs, and sections analyzed are specified in the figure legends.
ATP measurement
Whole explants or axonal samples derived from Lkb1 KO, Efhd1 KO, and WT inserts were collected in 100 mM Tri and 4 mM EDTA, pH 7.75, and incubated for 2 min at 90°C. ATP concentration was measured using ATP Bioluminescence Assay Kit CLS II (11699695001; Roche). Whole protein level in the same samples were quantified (BCA protein assay kit; Pierce). The ATP measurements were then normalized to the protein concentration.
Antibodies
Anti-tubulin βIII antibody (clone Tuj1; R&D Systems), at 1:20,000 for Western blot, 1:1,000 for immunoistochemistry (IHC) staining. P-AMPK thr172: phospho-AMPK-α (Thr172) (40H9) Cell Signaling, at 1:1,000. AMPK: AMPK-α (23A3) Cell Signaling, at 1:1,000. P-ACC ser 79: phospho-ACC (Ser79) Cell Signaling, at 1:1,000. ACC: anti-acetyl coenzyme A carboxylase antibody [EP687Y], AB-ab45174 Abcam, at 1:2,000. P-ULK ser555: phospho-ULK1 (Ser555) (D1H4) Cell Signaling, at 1:1,000. Caspase-3-cleaved: cleaved caspase-3 (Asp175) Cell Signaling, at 1:200. Islet antibody: Developmental Studies Hybridoma bank 39.3F7, at 1:200. Tom20: Santa Cruz Tom20 (FL-145): sc 11415, at 1:1,000 for immunofluorescence (STORM analysis) and TOM20 (D8T4N) Cell Signaling at 1:1,000 for immunoblot. β-Actin: β-Actin (13E5) Cell Signaling, at 1:20,000. Efhd1 antibody was a kind gift from the laboratory of Yasuhiro Tomooka (Tokyo University of Science), at 1:1,000. Anti-neurofilament antibody 2H3, Developmental Studies Hybridoma bank, at 1:200.
Antimouse and antirabbit antibodies conjugated with Alexa 549, Alexa 488, or Alexa 647 fluorophores were used at 1:200 (Jackson ImmunoResearch Laboratories). Secondary antibodies for Western blotting: goat antimouse IgG-HRP (JIR 155-035) and goat antirabbit IgG-HRP (JIR 111-035) from Jackson, both at 1:5,000.
Stochastic optical reconstruction microscopy (STORM) imaging
DRGs from E13.5 embryos were plated on cell culture dish as described above.
Three-dimensional super-resolution images were recorded using a Vutara SR200 STORM microscope (Bruker) based on single-molecule localization biplane technology with 60× Olympus water-immersion objective (1.2 NA). Mitochondria (anti-TOM20 staining) labeled with AlexaFluor 647 were imaged using 640-nm excitation laser and 405-nm activation laser in an imaging buffer composed of 5 mM cysteamine, oxygen scavengers (7 μM glucose oxidase and 56 nM catalase) in 50 mM Tris with 10 mM NaCl, and 10% glucose at pH 8.0. Images were recorded using Evolve 512 EMCCD camera (Photometrics) with gain set at 50, frame rate at 50 Hz, and maximal power of 640 and 405 nm lasers set at 6 and 0.05 kW/cm2, respectively. The total number of frames acquired was typically 15,000. Data analysis was performed using Vutara SRX software, localized particles were subjected to threshold value (set to 5) that is defined in Vutara SRX as standard deviations above the frame background value, which is determined based on the mean value of the border pixels in each frame. Mitochondrial length was manually analyzed using the SRX Vutara Software.
Mitochondrial DNA quantification
DRGs were cultured in six-well chambers as described above. On the second day, the cultures were treated with FUDR (5-fluoro-2′-deoxyuridine, CAS 50-91-9, 100 nM; Merck) for 8 h. On the third day, FUDR was removed. On the fifth day, DNA was extracted with the MasterPure DNA purification kit (Cat. no. MCD85201; Epicentre), and quantitative real-time PCR was performed using SYBR Green (Cat. no. 4385612; Applied Biosystems). Expression levels were determined using the comparative cycle threshold (2−ΔΔCt) method. 18S ribosomal RNA served as housekeeping genes.
Primers used were as follows:
ND1: FWD: 5′-TGCACCTACCCTATCACTCA-3′
REV: 5′-GCTCATCCTGATCATAGAATGG-3′
ND1 and ND4: FWD: 5′-CACTAATGCTACTACCACTAACCTGACTATC-3′
REV: 5′-TGTCATAGAAGTGTTAGGCTGGTTAA AC-3′
Cytb1: FWD: 5′-ACG TCC TTC CAT GAG GAC AA-3′
REV: 5′-GAG GTG AAC GAT TGC TAG GG-3′
COX1: FWD: 5′-GCCTTTGCTTCAAAACGAGA-3′
REV: 5′-GGTTGGTTCCTCGAATGTGT-3′
MT-ATP8: FWD: 5′-GCCACAACTAGATACATCAACATGA-3′
REV: 5′-GGTTGTTAGTGATTTTGGTGAAGGT-3′
18S: FWD-5′-AAACGGCTACCACATCCAAG-3′
REV-5′-CCTCCAATGGATCCTCGTTA-3′
Mitochondrial respiration studies (Seahorse)
For the mitochondrial respiration studies, DRGs were dissected as described above, washed in HBSS, and dissociated by incubation in trypsin–EDTA solution B (03-052-1B; Biological Sciences) for 5 min. After trypsinization, dissociated DRGs were suspended in L15 plus serum and plated at a concentration of 30 × 104 on PDL/laminin (described above)–coated XF 96 plates (102601-100; Agilent) with neurobasal (described before). On the second day, FUDR (100 nm) was added to the media for 4 h to eliminate dividing cells, thus obtaining a purer neuronal culture. On day 4, mitochondrial respiration was examined using the Seahorse XF96 analyzer (Agilent) and the XF Cell Mito Stress Test Kit according to the manufacturer’s instructions and as described in Karkucinska-Wieckowska et al (2015), Ruggiero et al (2017), Styr et al (2019). The analysis was performed in two parallel experiments as described in Styr et al., 2019. Briefly, in the first, respiration was measured under basal conditions and in response to the ATP synthases inhibitor oligomycin (1 μM), whereas in the second, respiration was measured under basal condition and in the presence of the electron transport chain accelerator ionophore FCCP (trifluorocarbonylcyanide phenylhydrazone, 4 μM). In both experiments, respiration was stopped by addition of the electron transport chain inhibitors rotenone and antimycin A (both at 0.5 μM). The neurons were then fixed with 4% formaldehyde and stained with tubulin βIII antibody. The plates were imaged, and the number of neurons was recalculated. Respiration values were normalized to the number of neurons. For each condition (oligomycin and rotenone/antimycin, and FCCP and rotenone/antimycin), we preformed four independent experiments; at least nine wells were used for each genotype, in each experiment. The baseline graph was generated by analyzing the third value of the baseline, in all the experiments. The ATP production graph was generated by subtracting the first value after oligomycin treatment from the third value of the baseline, in all the experiments. The SRC graph was generated by subtracting the third baseline value from the first value after FCCP injection, in all the experiments. The proton leak graph was generated by subtracting the first value after the injection of rotenone and antimycin from the third value after oligomycin injection, in all the experiments.
Mitochondrial motility
E13.5-dissociated DRGs (described above) from Lkb1 and Efhd1 KOs and their respective control neurons were plated on PDL/laminin–coated microfluidic chambers. After 72 h, the mitochondria were labeled by 5 nM TMRE (tetramethylrhodamine ethyl ester; Thermo Fisher Scientific) in the Lkb1 KO experiments and MitoTracker (M7514; Thermo Fisher Scientific) in the Efhd1 KO experiments and imaged for 6 h. Motility analyses were performed by ImageJ as described in Ionescu et al (2016).
Statistical analysis
Statistical analysis was performed using GraphPad Prism 7.0 software, Mean ± SEM is presented. The number of experiments (N) is indicated the figure legends. Normality of the data was defined using the Shapiro–Wilk normality test. For non-normally distributed data, Wilcoxon signed rank test and Mann–Whitney test were performed. For normally distributed homoscedastic data. two-tailed Student’s t test was used and for non-homoscedastic t test with Welch’s correction. For the Seahorse analysis, two-way ANOVA test and Sidak’s multi-comparison test were performed.
Supplementary Material
Acknowledgements
We thank the Yaron lab members for advice and criticism, Eran Perlson and Tal Pery Gradus for the mitochondrial motility analysis, Ruggiero Antonella for consultation and advise on the Seahorse experiments and analysis, Vladimir Kiss for the help with the confocal microscope, Ron Rotkopf for excellent statistical assistance, and Andrew Kovalenko for critically reading the manuscript. This work was supported by funding to A Yaron from The Legacy Heritage Biomedical Science Partnership of the Israel Science Foundation (1004/09), National Science Foundation (NSF-IOS/BOI grant [1556968]), and The Nella and Leon Benoziyo Center for Neurological Diseases; Mr and Mrs James Kelly, Helene T Fox, Daniel C Andreae, the Samuel Aba, and Sisel Klurman Foundation in honor of Prof. Joel Sussman; the Advantage Trust in honor of Prof. Joel Sussman; and the Estate of Ethel Lena Levy. A Yaron is an incumbent of the Jack and Simon Djanogly Professorial Chair in Biochemistry.
Author Contributions
V Ulisse: conceptualization, data curation, investigation, methodology, and writing—original draft, review, and editing.
S Dey: data curation and formal analysis.
DE Rothbard: data curation and investigation.
E Zeevi: data curation and investigation.
I Gokhman: data curation and investigation.
T Dadosh: data curation and investigation.
A Minis: conceptualization, data curation, and formal analysis.
A Yaron: conceptualization, resources, supervision, funding acquisition, project administration, and writing—original draft, review, and editing.
Conflict of Interest Statement
The authors declare that they have no conflict of interest.
References
- Alexander A, Walker CL (2011) The role of LKB1 and AMPK in cellular responses to stress and damage. FEBS Lett 585: 952–957. 10.1016/j.febslet.2011.03.010 [DOI] [PubMed] [Google Scholar]
- Barnes AP, Lilley BN, Pan YA, Plummer LJ, Powell AW, Raines AN, Sanes JR, Polleux F (2007) LKB1 and SAD kinases define a pathway required for the polarization of cortical neurons. Cell 129: 549–563. 10.1016/j.cell.2007.03.025 [DOI] [PubMed] [Google Scholar]
- Charron F, Stein E, Jeong J, McMahon AP, Tessier-Lavigne M (2003) The morphogen sonic hedgehog is an axonal chemoattractant that collaborates with Netrin-1 in midline axon guidance. Cell 113: 11–23. 10.1016/s0092-8674(03)00199-5 [DOI] [PubMed] [Google Scholar]
- Courchet J, Lewis TL, Lee S, Courchet V, Liou DY, Aizawa S, Polleux F (2013) XTerminal axon branching is regulated by the LKB1-NUAK1 kinase pathway via presynaptic mitochondrial capture. Cell 153: 1510–1525. 10.1016/j.cell.2013.05.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germain M, Nguyen MG, Khacho M, Patten DA, Screaton RA, Park DS, Slack RS (2013) LKB1-regulated adaptive mechanisms are essential for neuronal survival following mitochondrial dysfunction. Hum Mol Genet 22: 952–962. 10.1093/hmg/dds500 [DOI] [PubMed] [Google Scholar]
- Gwinn DM, Shackelford DB, Egan DF, Mihaylova MM, Mery A, Vasquez DS, Turk BE, Shaw RJ (2008) AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell 30: 214–226. 10.1016/j.molcel.2008.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie DG. (2007) AMP-activated/SNF1 protein kinases: Conserved guardians of cellular energy. Nat Rev Mol Cell Biol 8: 774–785. 10.1038/nrm2249 [DOI] [PubMed] [Google Scholar]
- Hardie DG. (2011) AMP-activated protein kinase-an energy sensor that regulates all aspects of cell function. Genes Dev 25: 1895–1908. 10.1101/gad.17420111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley SA, Boudeau J, Reid JL, Mustard KJ, Udd L, Mäkelä TP, Alessi DR, Hardie DG (2003) Complexes between the LKB1 tumor suppressor, STRAD alpha/beta and MO25 alpha/beta are upstream kinases in the AMP-activated protein kinase cascade. J Biol 2: 28 10.1186/1475-4924-2-28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou T, Jian C, Xu J, Huang AY, Xi J, Hu K, Wei L, Cheng H, Wang X (2016) Identification of EFHD1 as a novel Ca2+ sensor for mitoflash activation. Cell Calcium 59: 262–270. 10.1016/j.ceca.2016.03.002 [DOI] [PubMed] [Google Scholar]
- Ionescu A, Zahavi EE, Gradus T, Ben-Yaakov K, Perlson E (2016) Compartmental microfluidic system for studying muscle-neuron communication and neuromuscular junction maintenance. Eur J Cell Biol 95: 69–88. 10.1016/j.ejcb.2015.11.004 [DOI] [PubMed] [Google Scholar]
- Jiang P, Mizushima N (2015) LC3- and p62-based biochemical methods for the analysis of autophagy progression in mammalian cells. Methods 75: 13–18. 10.1016/j.ymeth.2014.11.021 [DOI] [PubMed] [Google Scholar]
- Karkucinska-Wieckowska A, Pronicki M, Wieckowski MR (2015) Histoenzymatic methods for visualization of the activity of individual mitochondrial respiratory chain complexes in the muscle biopsies from patients with mitochondrial defects. Methods Mol Biol 1241: 85–93. 10.1007/978-1-4939-1875-1_8 [DOI] [PubMed] [Google Scholar]
- Lilley BN, Pan YA, Sanes JR (2013) SAD kinases sculpt axonal arbors of sensory neurons through long- and short-term responses to neurotrophin signals. Neuron 79: 39–53. 10.1016/j.neuron.2013.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maor-nof M, Romi E, Shalom HS, Ulisse V, Raanan C, Nof A, Leshkowitz D, Lang R, Yaron A (2016) Axonal degeneration is regulated by a transcriptional program that coordinates expression of pro- and anti-degenerative factors. Neuron 92: 991–1006. 10.1016/j.neuron.2016.10.061 [DOI] [PubMed] [Google Scholar]
- Marvaldi L, Thongrong S, Kozłowska A, Irschick R, Pritz CO, Bäumer B, Ronchi G, Geuna S, Hausott B, Klimaschewski L (2015) Enhanced axon outgrowth and improved long-distance axon regeneration in sprouty2 deficient mice. Dev Neurobiol 75: 217–231. 10.1002/dneu.22224 [DOI] [PubMed] [Google Scholar]
- Maryanovich M, Zaltsman Y, Ruggiero A, Goldman A, Shachnai L, Zaidman SL, Porat Z, Golan K, Lapidot T, Gross A (2015) An MTCH2 pathway repressing mitochondria metabolism regulates haematopoietic stem cell fate. Nat Commun 6: 7901 10.1038/ncomms8901 [DOI] [PubMed] [Google Scholar]
- Misgeld T, Schwarz TL (2017) Mitostasis in neurons: Maintaining mitochondria in an extended cellular architecture. Neuron 96: 651–666. 10.1016/j.neuron.2017.09.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano A, Takashima S (2012) LKB1 and AMP-activated protein kinase: Regulators of cell polarity. Genes Cells 17: 737–747. 10.1111/j.1365-2443.2012.01629.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pooya S, Liu X, Kumar VBS, Anderson J, Imai F, Zhang W, Ciraolo G, Ratner N, Setchell KDR, Yutaka Y, et al. (2014) The tumour suppressor LKB1 regulates myelination through mitochondrial metabolism. Nat Commun 5: 4993 10.1038/ncomms5993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redmann M, Benavides GA, Berryhill TF, Wani WY, Ouyang X, Johnson MS, Ravi S, Barnes S, Darley-Usmar VM, Zhang J (2017) Inhibition of autophagy with bafilomycin and chloroquine decreases mitochondrial quality and bioenergetic function in primary neurons. Redox Biol 11: 73–81. 10.1016/j.redox.2016.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renier N, Wu Z, Simon DJ, Yang J, Ariel P, Tessier-Lavigne M (2014) IDISCO: A simple, rapid method to immunolabel large tissue samples for volume imaging. Cell 159: 896–910. 10.1016/j.cell.2014.10.010 [DOI] [PubMed] [Google Scholar]
- Rio DC, Are M, Hannon GJ, Nilsen TW (2010) Purification of RNA using TRIzol (TRI reagent). Cold Spring Harbor Protoc 5: 1–4. 10.1101/pdb.prot5439 [DOI] [PubMed] [Google Scholar]
- Rishal I, Golani O, Rajman M, Costa B, Ben-Yaakov K, Schoenmann Z, Yaron A, Basri R, Fainzilber M, Galun M (2013) WIS-neuromath enables versatile high throughput analyses of neuronal processes. Dev Neurobiol 73: 247–256. 10.1002/dneu.22061 [DOI] [PubMed] [Google Scholar]
- Rizzuto R, De Stefani D, Raffaello A, Mammucari C (2012) Mitochondria as sensors and regulators of calcium signalling. Nat Rev Mol Cell Biol 13: 566–578. 10.1038/nrm3412 [DOI] [PubMed] [Google Scholar]
- Romi E, Gokhman I, Wong E, Antonovsky N, Ludwig A, Sagi I, Saftig P, Tessier-Lavigne M, Yaron A (2014) ADAM metalloproteases promote a developmental switch in responsiveness to the axonal repellant Sema3A. Nat Commun 5: 1–15. 10.1038/ncomms5058 [DOI] [PubMed] [Google Scholar]
- Ruggiero A, Aloni E, Korkotian E, Zaltsman Y, Oni-Biton E, Kuperman Y, Tsoory M, Shachnai L, Levin-Zaidman S, Brenner O, et al. (2017) Loss of forebrain MTCH2 decreases mitochondria motility and calcium handling and impairs hippocampal-dependent cognitive functions. Sci Rep 7: 1–13. 10.1038/srep44401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackelford DB, Shaw RJ (2009) The LKB1-AMPK pathway: Metabolism and growth control in tumour suppression. Nat Rev Cancer 9: 563–575. 10.1038/nrc2676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw RJ, Kosmatka M, Bardeesy N, Hurley RL, Witters LA, DePinho RA, Cantley LC (2004) The tumor suppressor LKB1 kinase directly activates AMP-activated kinase and regulates apoptosis in response to energy stress. Proc Natl Acad Sci U S A 101: 3329–3335. 10.1073/pnas.0308061100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelly M, Cancedda L, Heilshorn S, Sumbre G, Poo MM (2007) LKB1/STRAD promotes axon initiation during neuronal polarization. Cell 129: 565–577. 10.1016/j.cell.2007.04.012 [DOI] [PubMed] [Google Scholar]
- Sheng ZH. (2017) The interplay of axonal energy homeostasis and mitochondrial trafficking and anchoring. Trends Cell Biol 27: 403–416. 10.1016/j.tcb.2017.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spillane M, Ketschek A, Merianda TT, Twiss JL, Gallo G (2013) Mitochondria coordinate sites of axon branching through localized intra-axonal protein synthesis. Cell Rep 5: 1564–1575. 10.1016/j.celrep.2013.11.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein M, Dütting S, Mougiakakos D, Bösl M, Fritsch K, Reimer D, Urbanczyk S, Steinmetz T, Schuh W, Bozec A, et al. (2017) A defined metabolic state in pre B cells governs B-cell development and is counterbalanced by Swiprosin-2/EFhd1. Cell Death Differ 24: 1239–1252. 10.1038/cdd.2017.52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Styr B, Gonen N, Zarhin D, Ruggiero A, Atsmon R, Gazit N, Braun G, Frere S, Vertkin I, Shapira I, et al. (2019) Mitochondrial regulation of the hippocampal firing rate set point and seizure susceptibility. Neuron 102: 1009–1024.e8. 10.1016/j.neuron.2019.03.04 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swisa A, Granot Z, Tamarina N, Sayers S, Bardeesy N, Philipson L, Hodson DJ, Wikstrom JD, Rutter GA, Leibowitz G, et al. (2015) Loss of liver kinase B1 (LKB1) in beta cells enhances glucose-stimulated insulin secretion despite profound mitochondrial defects. J Biol Chem 290: 20934–20946. 10.1074/jbc.m115.639237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tominaga M, Kurihara H, Honda S, Amakawa G, Sakai T, Tomooka Y (2006) Molecular characterization of mitocalcin, a novel mitochondrial Ca2+-binding protein with EF-hand and coiled-coil domains. J Neurochem 96: 292–304. 10.1111/j.1471-4159.2005.03554.x [DOI] [PubMed] [Google Scholar]
- Toyama EQ, Herzig S, Courchet J, Lewis TL Jr, Losón OC, Hellberg K, Young NP, Chen H, Polleux F, Chan DC, et al. (2016) AMP-activated protein kinase mediates mitochondrial fission in response to energy stress. Science 351: 275–281. 10.1126/science.aab4138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tymanskyj SR, Yang BH, Verhey KJ, Ma L (2018) MAP7 regulates axon morphogenesis by recruiting kinesin-1 to microtubules and modulating organelle transport. Elife 7: 1–27. 10.7554/elife.36374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaarmann A, Mandel M, Zeb A, Wareski P, Liiv J, Kuum M, Antsov E, Liiv M, Cagalinec M, Choubey V, et al. (2016) Mitochondrial biogenesis is required for axonal growth. Development 143: 1981–1992. 10.1242/dev.128926 [DOI] [PubMed] [Google Scholar]
- Zhang X, Garbett K, Veeraraghavalu K, Wilburn B, Gilmore R, Mirnics K, Sisodia SS (2012) A role for presenilins in autophagy revisited: Normal acidification of lysosomes in cells lacking PSEN1 and PSEN2. J Neurosci 32: 8633–8648. 10.1523/jneurosci.0556-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang CS, Lin SC (2016) AMPK promotes autophagy by facilitating mitochondrial fission. Cell Metab 23: 399–401. 10.1016/j.cmet.2016.02.017 [DOI] [PubMed] [Google Scholar]
- Zylka MJ, Rice FL, Anderson DJ (2005) Topographically distinct epidermal nociceptive circuits revealed by axonal tracers targeted to Mrgprd. Neuron 45: 17–25. 10.1016/j.neuron.2004.12.015 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Source Data for Figure S1LSA-2020-00753_SdataFS1.pdf (6.5MB, pdf)
Source Data for Figure 1LSA-2020-00753_SdataF1.pdf (1.4MB, pdf)
Table S1 Gene expression analysis of WT and Lkb1 KO E13.5 DRGs.Gs. (5.2MB, xls)
Source Data for Figure S2LSA-2020-00753_SdataFS2.pdf (3.6MB, pdf)
Source Data for Figure S3LSA-2020-00753_SdataFS3.pdf (6.8MB, pdf)
Source Data for Figure 5LSA-2020-00753_SdataF5.pdf (8.8MB, pdf)