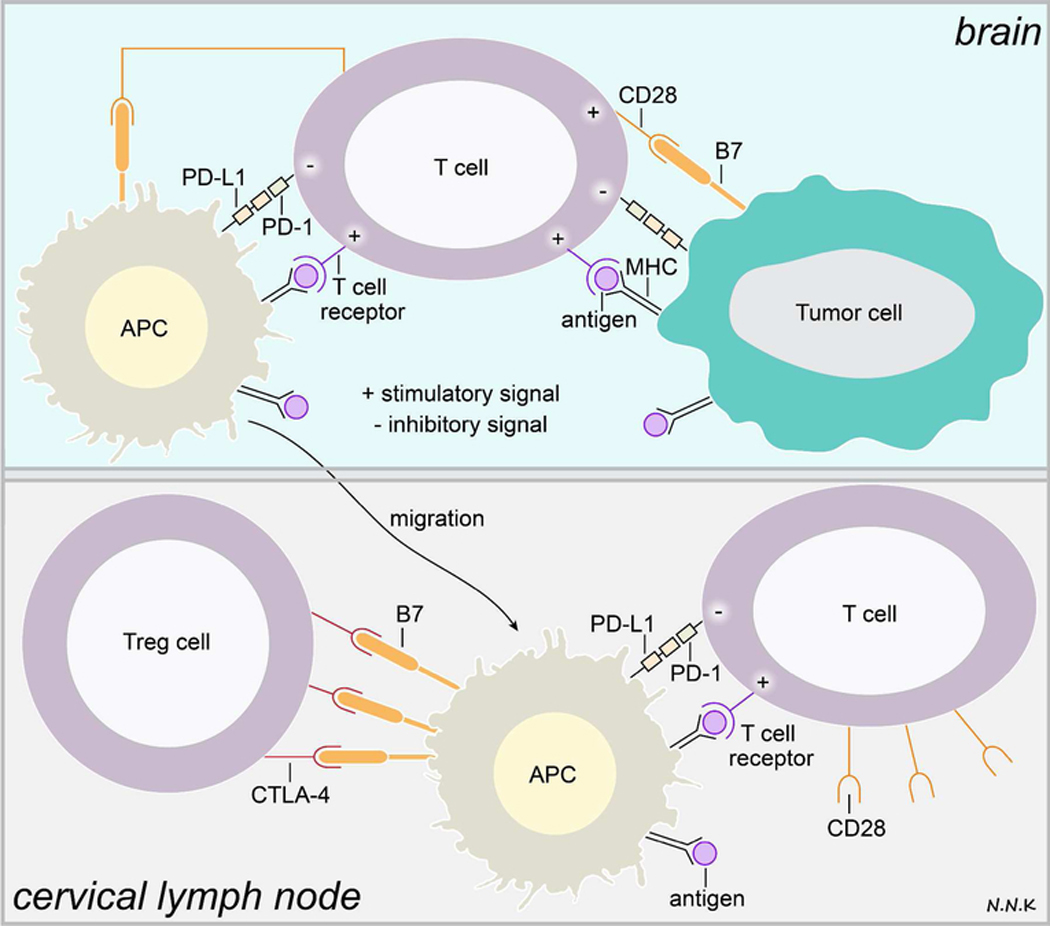
Figure 3. T cell immune response and immune checkpoints in brain cancer.
T cells may recognize tumor antigen peptides presented via MHC class I/II molecules on tumor cells or antigen presenting cells (APCs) via the TCR, resulting in a weak immune stimulatory signal. Interaction between the TCR and tumor antigen peptide/MHC complex can only activate the T cell in the presence of other co-stimulatory immune signaling. However, tumor cells and APCs in the tumor microenvironment express high levels of programmed cell death-ligand-1 (PD-L1), a ligand for the programmed cell death - (PD-1) receptor expressed by T cells, which inhibits T cell activation. APCs presenting the tumor antigen peptide/MHC complex may migrate to the cervical lymph nodes where T cells recognizing the tumor antigen may be activated and directed to the tumor. In addition to the TCR-tumor antigen/MHC interaction, the T cell must receive co-stimulatory signals in order to be activated. This co-stimulatory signal is typically received when the classification determinant 28 (CD28) receptor on T cells interacts with the B7 ligand expressed by APCs. However, regulatory T cells (Treg cells) express high amounts of cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) – a receptor that mimics CD28 and has an even higher affinity for the B7 ligand. Thus, CTLA-4-B7 interaction can compete with the CD28-B7 interaction, resulting in the lack of appropriate co-stimulatory signaling to activate tumor antigen recognizing T cells. Adapted from: 53–55. Abbreviations: PD-1 – programmed cell death protein-1; PD-L1 – programmed cell death protein ligand-1; CTLA-4 – cytotoxic T-lymphocyte-associated protein 4; Treg – regulatory T cells; CD28 – classification determinant 28.