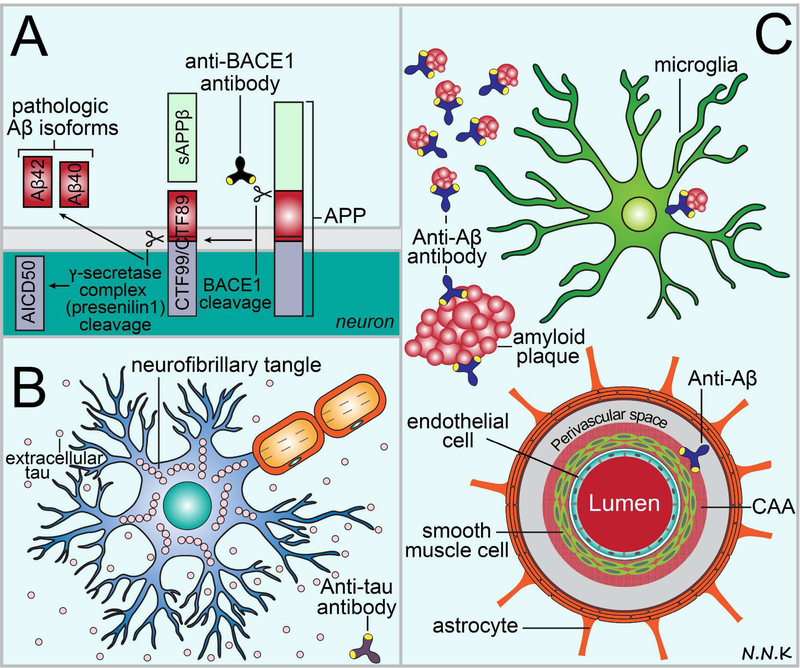
Figure 8.
Passive immunotherapy strategies to treat Alzheimer’s disease (AD). Some of the major hallmarks of pathology in AD include: (i) excess production of amyloid β-peptide (Aβ) fragments catalyzed by the beta-site APP-cleaving enzyme 1 (BACE1) and γ-secretase enzyme complex cleavage of the amyloid precursor protein (APP); (ii) accumulation and aggregation of hyperphosphorylated tau within neurons leading to cell death and cell-to-cell transmission of extracellular tau; and (iii) accumulation and aggregation of Aβ within the brain parenchyma (Aβ42) and the perivascular compartments of cerebral arteries (Aβ40). Passive immunotherapy may be used to target these different features of AD pathology. (A) Anti-BACE1 antibodies can be used to block the BACE1 cleavage of APP and thus minimize abnormal and excess production of Aβ fragments. (B) Anti-tau antibodies that target hyperphosphorylated tau can be used to block intracellular tau aggregation (likely using intrabodies 169) and prevent the extracellular cell-to-cell transmission of pathologic tau (conventional antibodies 163,164). (C) Anti-Aβ42 antibodies can be used to target Aβ42 in the brain parenchyma and halt or reverse disease pathology by aiding microglia mediated Aβ42 clearance via Fc interactions, binding to monomers and oligomers and preventing their aggregation, and resolving plaques via serine protease activity. Anti-Aβ40 antibodies may be used to target Aβ40 accumulation in the perivascular compartment of cerebral arteries (also referred to as cerebral amyloid angiopathy or CAA) in a similar manner. Adapted from: 136,183–185.