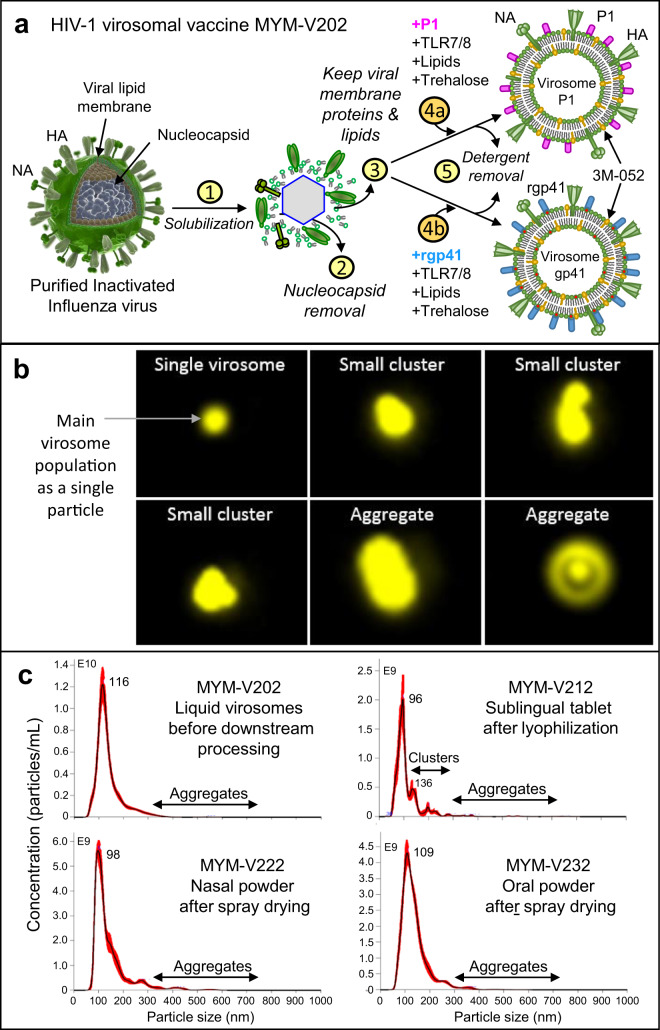
Fig. 1. Influenza virosome-based vaccine manufacturing.
a Production of adjuvanted virosome-P1 (MYM-V111) and virosome-rgp41 (MYM-V112): Step 1, inactivated influenza A/H1N1 are solubilized with detergent; Step 2, nucleocapsides are discarded; Step 3, the viral membrane lipids with the native influenza hemagglutinin (HA) and neuraminidase (NA) are recovered; Steps 4a and 4b, synthetic lipids with 3M-052 adjuvant and antigen P1 or rgp41 are mixed with isolated viral membrane components and trehalose; Step 5, virosomes-P1 (pink rod) and virosome-rgp41 (blue rod) are gradually assembled in vitro during the detergent removal. Each virosome is then diluted and mixed together to generate the HIV-1 liquid vaccine MYM-V202. Universal T help provided by HA/NA. b Amnis® ImageStream on fluorescent Dil dye-labeled virosomes (in yellow) to visualize particles. The liquid virosome population contains mostly single particles (upper left image). Reconstituted powders contain also a major population of single particles, but a minor population of few small virosome clusters and bigger aggregates are also observed. Images were enlarged in Powerpoint because original AMNIS images are only tiny dots. c Mean particle size and population distribution monitored by NTA for the liquid bulk vaccine (MYM-V202, upper left panel), the reconstituted sublingual tablet (MYM-V212, upper right panel), the reconstituted nasal powder (MYM-V222, lower left panel), the reconstituted oral powder (MYM-V232, lower right panel). Black arrows identify the population of small clusters 200–300 nm and aggregates >300 nm, using arbitrary cut-off.