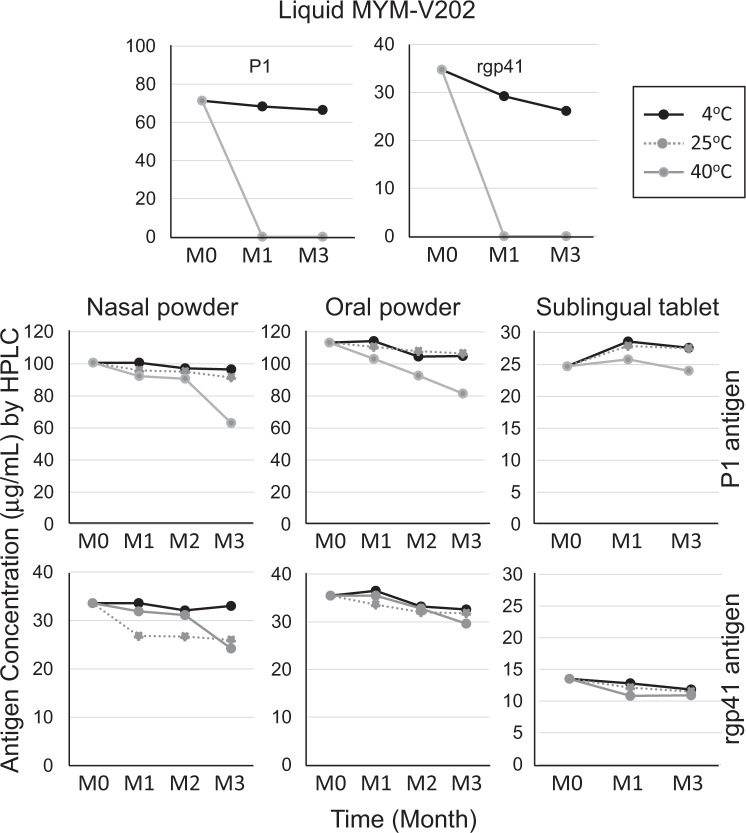
Fig. 6. Antigen concentrations in the various vaccine forms exposed to different environmental conditions.
The liquid adjuvanted vaccine formulation MYM-V202 containing both P1 and rgp41 antigens served as reference material for comparison to the solid vaccine dosage form for nasal, oral, and sublingual delivery. A 3 months stability study was performed on the various liquid and solid vaccine forms stored under three different temperatures and relative humidity (RH) conditions: 4 °C (black line), 25 °C/65% RH (gray dot line, not done for the liquid form), and 40 °C/75% RH (gray line). P1 and rgp41 antigens were previously shown to be temperature sensitive, which is confirmed again here in the first two upper panels, showing rapid P1 and rgp41 modifications at 40 °C, as compared to the liquid vaccine stored under the recommended temperature at 4 °C. For solid vaccine forms, at each indicated month time point (M0, M1, M2, or M3), samples were reconstituted with water and analyzed by HPLC for the P1 and rgp41 content. Note that chemical modifications such as oxidation or deamidation on antigens are the main reasons to the observed lower antigen concentration, as antigen degradation could not be reported by native immunoblot. Data shown are from representative HPLC measures.