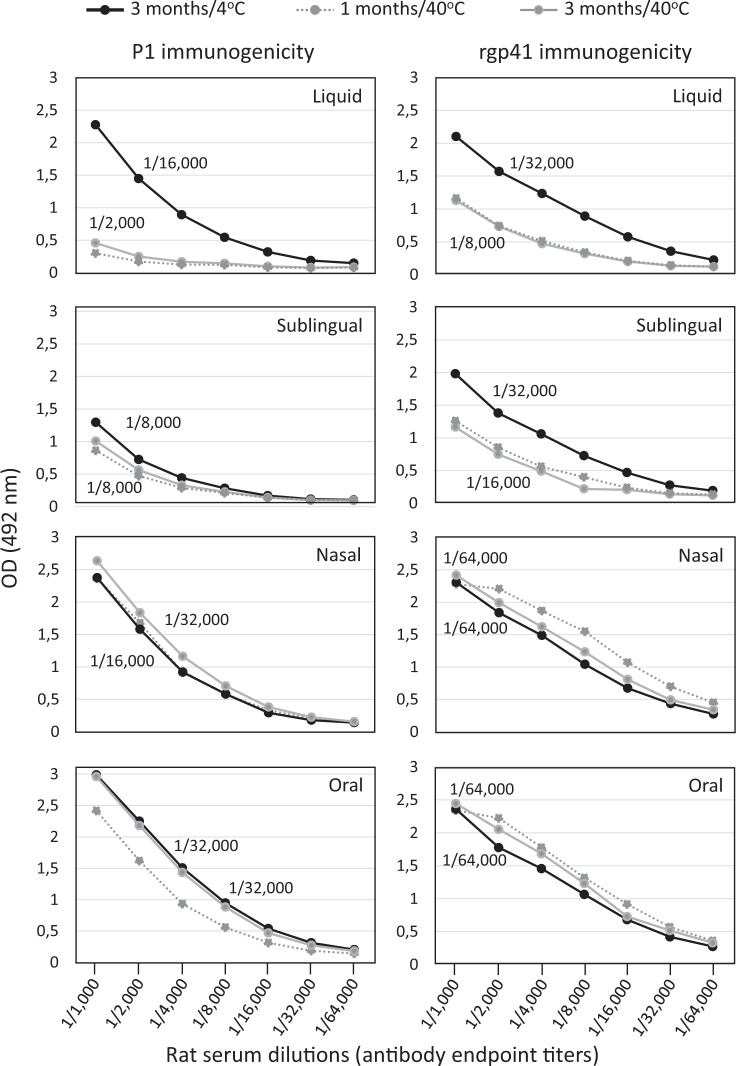
Fig. 7. Immunogenicity of P1 and rgp41 from liquid and various solid vaccine dosage forms exposed to different temperatures.
The liquid adjuvanted vaccine formulation MYM-V202 containing both P1 and rgp41 antigens were temperature sensitive and served as reference material for comparison with the immunogenicity of the solid vaccine forms with improved thermostability. Black line, vaccines stored 3 months at 2–8 °C; gray dot line, vaccines exposed 1 month at 40 °C; gray line, vaccines exposed 3 months at 40 °C. In each panel, the antibody endpoint titers (specific toward P1 or rgp41 antigen) of the serum pool of 10 rat sera are indicated, data are from a representative experiment. Endpoint titer against each antigen corresponds to the last serum dilution generating an optical density (OD) value >2-fold above the pre-immune background.