Abstract
Fibrous dysplasia (FD) is a rare condition commonly involving the jaws. While FD has a typical clinical and histological presentation, considerable variation exists. Moreover, overlap of features with other disorders is possible. This study serves to characterize the features of a large case series of FD of the jaws. With IRB approval, the University of Florida Oral Pathology Biopsy Service archive was retrospectively searched from 1994 to 2015 for cases of FD. Epidemiological data, location, duration, clinical and radiographic appearance, clinical impression and exact microscopic diagnosis were recorded. The average age was 37.3 years (range 7–87 years) with majority of cases in females (67.5%). The most common ethnicity was Caucasian. Maxillary location was predominant (59%), followed by mandible (38%) and multiple locations (3%). Expansion was reported in 78% of cases. Radiographically, most cases exhibited ground glass opacity, however some presented with a mottled or mixed radiopaque/radiolucent appearance. Histologically, a wide variation in terms of stromal cellularity, presence of osteoblastic rimming, and presence of calcified material mimicking cemento-osseous dysplasia was observed. Clinicians and pathologists should be cognizant of the significant variability in clinical, histopathologic, and radiographic presentation of FD, which may pose a diagnostic challenge.
Keywords: Fibrous dysplasia, Mandible, Maxilla, Gnathic, Fibro-osseous
Introduction
Fibrous dysplasia (FD) is a benign genetic developmental condition characterized by the replacement of normal bone with fibrous stroma and structurally weak immature bone. FD is a rare condition which often involves the jaws. Usually FD presents with typical clinical and histological presentation, considerable variations still exist. Moreover, FD may present with features that may overlap with other bone conditions/lesions.
Past studies of FD have mainly been aimed at better delineation of characteristic clinicopathologic features of gnathic and cranio-facial FD. While the knowledge of classic clinicopathologic characteristics of FD is necessary for proper diagnosis, recognition of the spectrum of clinical, radiographic and histopathologic presentations is also an important part of the diagnostic process. Lack of familiarity with the less common presentations of FD may possibly lead to misdiagnosis. The aim of this study was to analyze and characterize the various demographic, clinical, radiographic and histologic features of FD of the jaws from a large case series.
Materials and Methods
A retrospective search of the University of Florida Oral Pathology Biopsy Service archive was performed after receiving an IRB approval. The archive was searched from January 1, 1994, through July 1, 2015, for cases signed out as benign fibro-osseous lesion consistent with fibrous dysplasia. The original biopsy slides were retrieved, and all the specimens were reviewed for inclusion criteria and agreement with final diagnosis rendered.
Cases not meeting clinical, radiographic, or histologic diagnostic criteria for FD as defined by the most recent WHO Classification were excluded. The WHO classification described typically as expansile lesions which may appear radiolucent or variably opaque often with a “ground-glass” appearance and histologically demonstrating the presence of irregular trabecular bone surrounded by bland fibroblastic connective tissue [1]. Cases with insufficient tissue, including fragmented biopsies, small biopsies, poorly processed specimens, and multiple subsequent biopsies from the same patient were excluded from our study.
For each case the epidemiological data, location, duration, clinical and radiographic appearance, as well as clinical impression and microscopic diagnosis was recorded and subsequently analyzed.
The histologic features of FD involving of the jaws have been extensively evaluated in prior literature and were reviewed here by two investigators (SF and LD) [1–12]. These included presence or absence of the following features: lamellar bone, resting and reversal lines, artifactual peri-trabecular clefting, osteoblastic rimming, cementoid bodies, and the presence of smooth transition to surrounding normal bone.
Results
Demographics
Forty cases fit the search criteria and were included in this study. The average age at presentation in our study was 37.3 years (range 7–87 years). Case distribution based on gender showed that females were affected more than males (67.5% vs. 32.5%), approximately 2:1. Out of 33 cases where race was reported the majority were Caucasians, n = 19, followed by African Americans, n = 11, with only two Asian and one Hispanic patient.
Clinical Presentation
A unilateral presentation was noted in the majority of patients in this study (n = 38). 23 cases were reported in the maxilla, with 13 on the left and 10 on the right. Mandibular involvement was noted in 15 patients with equal distribution between the left (n = 7) and right (n = 7) sides. The exact location for one of the mandibular lesions was not specified. In addition, multiple sites were affected in one of the cases, and no specific location was provided in one case.
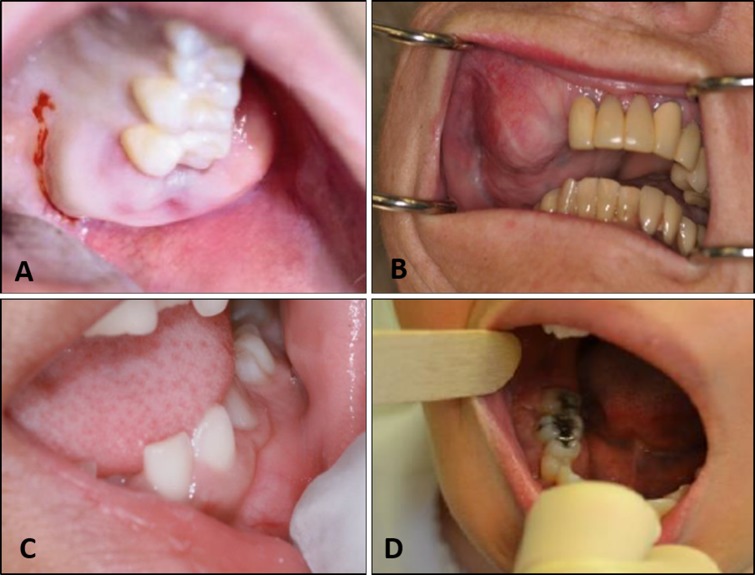
Clinical symptoms were reported for 37 patients out of 40. Expansion alone was noted in 31 of the cases. In two cases it was reported in combination with pain, and one each with tooth mobility and pain and tooth mobility alone. Two cases were completely asymptomatic. The rarity of pain associated with FD of the jaw is noteworthy. Some of the diversity of clinical appearances is illustrated in Fig. 1.
Fig. 1.
Clinical spectrum of reported fibrous dysplasia cases. a Diffuse expansion of left maxilla (image courtesy Dr. Richard Glow), b extensive expansion of the right maxilla in a longstanding lesion of an elderly patient, c mild expansion in the left mandible in a pediatric patient (image courtesy Dr. Thomas Porter), d expansion of the right mandible (image courtesy Dr. Luis Rosario)
The duration of symptoms was reported in 24 cases. Symptoms were present for less than 6 months (n = 4), between 6 months and 1 year (n = 2), between one and 5 years (n = 14), and for over 5 years (n = 4). Symptom duration was not listed for the remaining 16 cases.
The clinical impression was provided by the submitting clinicians for 36 out of 40 lesions. FD was suspected in only 13 cases. Benign fibro-osseous lesions (BFOL) such as cemento-ossifying fibroma (COF) and cemento-osseous dysplasia (COD) were suspected in 12 cases. Clinical impression of exostosis or dense bone was noted in 3 cases, while odontogenic cysts and tumors such as myxoma, calcifying epithelial odontogenic tumor (CEOT) and ameloblastoma were anticipated in 2 cases. Clinical differential diagnoses such as osteosarcoma, central giant cell granuloma (CGCG), cherubism, osteoma, and osteoblastoma were indicated in the other 2 cases. Paget’s disease was a primary differential for FD in 2 cases. Salivary gland tumors, lymphoma, and desmoplastic fibroma were suspected in 2 patients. No clinical impression was mentioned for the remaining 4 lesions.
Radiographic Presentation
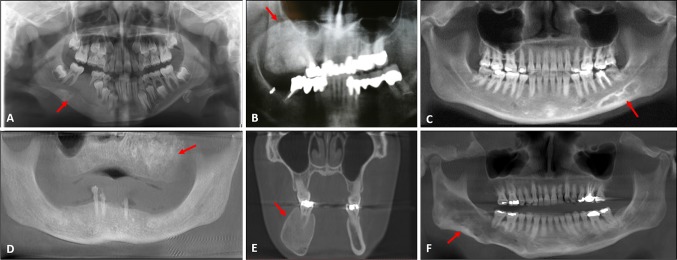
23 radiographic images were available for analysis in this study but one of the radiographs was uninterpretable due to low quality. The vast majority of cases (18/22) had a ground glass appearance with or without expansion. The mandibular lesions often presented with enhanced trabeculations rather than a diffuse ground glass appearance. The radiographic appearances of FD are summarized in Table 1. Some examples of the variation in radiographic appearance from cases in this series can be seen in Fig. 2.
Table 1.
Summary of radiographic appearances
| Radiographic description | Number of cases |
|---|---|
| Ground glass RO with expansion | 15 |
| Ground glass RO alone | 3 |
| Mixed RL/RO | 2 |
| Soap bubble RL | 1 |
| Mottled expansile RO | 1 |
| Mixed multilocular lesion with corticated margins | 1 |
| Additional features | |
| Root resorptiona | 3 |
| Inferior alveolar canal displacement (inferiorly)a | 1 |
RO radiopacity, RL radiolucency
aThese features were seen in addition to ground glass RO and expansion
Fig. 2.
Radiographic spectrum of reported fibrous dysplasia cases. Areas of interest highlighted with red arrows. a Ground glass expansile radiopacity in the right mandible (radiograph courtesy Dr. Pat Ricalde). b Extensive expansile radiopacity in the right maxilla (clinical photo in Fig. 1b), c sclerosis and mixed radiopacity in the left mandible (radiograph courtesy Dr. Scott Wenk), d diffuse ground glass expansile radiopacity in the edentulous maxilla (radiograph courtesy Dr. Ronald Caylor), e diffuse enlargement of the right mandible (radiograph courtesy Dr. Luis Rosario), f cotton-wool expansive radiopacity in the right mandible demonstrating bowing of the inferior border of the mandible (image courtesy Dr. Corey Auch)
Histologic Evaluation
Though all cases fit histologic criteria for FD, wide variation was noted regarding histologic features such as stromal cellularity, the presence of osteoblastic rimming, and the presence of calcified material mimicking cemento-osseous dysplasia. Individual histologic features were assessed for each case and the results are summarized in Table 2. Though many cases appeared consistent with classic histologic features of FD (Fig. 3a and b), some showed signs of maturation of the lesion such as increased amounts of lamellar bone. Cellularity of the fibrous stroma varied from sparse to highly cellular (Fig. 3c and d). Bone deposition ranged from Pagetoid-appearing with prominent resting and reversal lines to areas mimicking cemento-osseous dysplasia with focal areas of small cementoid bodies noted in some cases (Fig. 3e and f). Osteoblastic rimming was also variable between cases (Fig. 3g and h).
Table 2.
Extent of the histological features present in FD cases (total n = 40)
| Lamellar bone | Resting/reversal lines | Peritrabecular clefting | Osteoblastic rimming | Cementoid bodies | Transition to normal bone | |
|---|---|---|---|---|---|---|
| Absent | 21 (52.5%) | 21 (52.5%) | 3 (7.5%) | 23 (57.5%) | 31 (77.5%) | 27 (67.5%) |
| Focal (less than 25%) | 8 (20%) | 12 (30%) | 3 (7.5%) | 14 (35%) | 7 (17.5%) | 0 (0%) |
| Moderate (25–50%) | 1 (2.5%) | 4 (10%) | 3 (7.5%) | 2 (5%) | 1 (2.5%) | 0 (0%) |
| Generalized (50–90%) | 6 (15%) | 1 (2.5%) | 21 (52.5%) | 1 (2.5%) | 0 (0%) | 0 (0%) |
| Diffuse (over 90%) | 4 (10%) | 2 (5%) | 10 (25%) | 0 (0%) | 1 (2.5%) | 13 (32.5%) |
Fig. 3.
Histologic variation in reported fibrous dysplasia cases (all images hematoxylin and eosin stain). a Low power magnification demonstrating classic FD histologic appearance with curvilinear islands of bone demonstrating retraction artifact in a background of fibrous connective tissue, b presentation of a longstanding or “mature” lesion (low power magnification), c specimen with high level of cellularity (low power magnification) and d low level of cellularity (medium magnification), e specimen demonstrating pagetoid-like areas (medium magnification) and f cemento-osseous dysplasia-like areas (low power magnification), g lesion with focal areas of osteoblastic rimming evident (high power magnification) and h absent osteoblastic rimming (high power magnification)
Discussion
Fibrous dysplasia (FD) is a benign developmental genetic non-inheritable condition characterized by the replacement of normal bone with fibrous stroma and structurally weak immature bone. This lesion falls under a broader category of conditions known as benign fibro-osseous lesions (BFOL) [1, 3, 5, 6]. These lesions have similar microscopic features but unique clinical and radiographic characteristics.
Etiology
FD is etiologically associated with post-zygotic point mutation of the GNAS gene located on the long arm of chromosome 20 at the Arg201 codon (20q 13.2–13.3) and involves the stimulatory G-nucleotide binding protein α-subunit (Gsα) [1, 2, 5, 11, 13, 14]. The mutation results in the activation of adenylyl cyclase and increased 3′–5′-cyclic adenosine monophosphate production (c-CAMP), and consequently an aberrant osteoblastic cell differentiation [1, 5, 14, 15].
The extent of the bone involvement (focal vs. generalized) depends on the timing of mutation during the embryogenesis. Earlier mutations lead to a more generalized and more severe manifestation of the disease (polyostotic and syndromic), while later mutations result in focal or monostotic bone involvement [2, 16–18].
Mutation of pluripotent stem cells during early embryogenesis affects melanocytes, endocrine cells, and osteoblasts and subsequently manifests as fibrous dysplasia associated with a syndrome [19]. Skeletal stem cell mutations that occur during later embryonic development lead to abnormalities in osteoblasts only. Finally, postnatal mutations result only in a single bone involvement [20]. The activation of Gsα protein impairs osteoblastic differentiation and results in an inappropriate osteoblastic cell proliferation and differentiation. An excessive production of fibrous connective tissue with bony trabeculae ensues. In addition, Gsα protein has a stimulating effect on endocrine cells and melanocytes [5, 14, 15].
Clinically, there are three variants of FD of the jaws: monostotic, polyostotic, and syndromic [6, 8]. Such classification may reflect the timing of the mutation [2]. Monostotic FD involves one bone, polyostotic FD involves more than one bone, and syndromic involves FD associated with one of the three syndromes. Jaffe-Lichtenstein syndrome is characterized by polystotic FD with café-au-lait cutaneous spots; Mazabraud syndrome consisting of FD and intramuscular myxomas [2, 17, 21]; and McCune-Albright syndrome in which FD is seen in combination with café-au-lait cutaneous spots and endocrinopathies [1–3, 6–8, 19, 21].
Besides the aforementioned forms of FD, an additional variant exists which does not precisely fit into the criteria of either monostotic or polyostotic FD. This specific type is termed craniofacial FD and it affects primarily the maxilla and its contiguous bones, such as sphenoid, zygomatic, frontonasal, and base of the skull bones [1–4, 7, 16, 18].
Clinical Presentation
The mean age for skeletal FD is 15 years. While for gnathic FD investigators report different mean ages, it generally affects an older population. The mean age in the study by Netto et al. is 34.5 (age range 11–65) [15]. Alsharif et al. reported a similar mean age of 33.2 (age range 10–69) [18]. A clinicopathological analysis by Phattarataratip et al. shows a lower mean age of 24.9 (age range 7–62) [22] and a systematic review by MacDonald-Jankowski was in significant agreement with the mean age of FD of 24 years [23]. The average age at presentation in the present study was 37.3 years (range 7–87 years), which is even older than what is reported in the literature. Interestingly most literature reports that FD of the jaws most commonly arises in the 2nd and 3rd decades [10, 18, 22–24]. The reason for this discrepancy is the subtle nature of presentation of many jaw lesions leading to later discovery and/or initial misdiagnosis or almost complete lack of symptoms.
In terms of gender predilections, our findings were similar to those found in the current literature showing approximately a 2:1 ratio of female to male ratio. The posterior maxilla was the most commonly affected site for gnathic FD, a finding also consistent with the literature. These lesions typically present as a painless expansion [3–8, 22–24].
Clinical differentials for FD include ossifying fibroma (COF), segmental odontomaxillary dysplasia, familial gigantiform cementoma (FGC), and Paget disease of bone. The distinction between those entities is made based on radiographic and histologic findings in combination with laboratory test results. COF is exclusive to tooth-bearing areas of the jaws and has predilection for posterior mandible. It typically presents as a well-circumscribed radiolucency. Histologically, the pattern of mineralization in COF is variable in contrast to uniform mineralization seen in FD [2]. SOD typically presents during childhood. Usually one or two maxillary premolars fail to errupt in the affected area and overlying gingiva is hyperplastic. Radiographically it is characterized by thickened and vertically oriented trabeculae appearing as a “sleet storm” [5]. FGC is a rare entity that presents as a multifocal progressively expansile lesion of early onset [25]. Paget disease affects an older population, is usually associated with pain, and blood testing often reveals elevated levels of serum alkaline-phosphatase with normal levels of phosphorus and calcium [26]. It however can occasionally be confused with late onset FD.
Classic Radiographic Presentation and Radiographic Variants
The radiographic features of head and neck FD vary based on the stage of the disease.
Earlier lesions present as a radiolucency while later lesions tend to be more sclerotic [1, 3–5, 7]. FD is often characterized as demonstrating a classic “ground glass” appearance. The lesion is generally diffuse and has ill-defined borders that merge imperceptibly with the surrounding normal bone [1–8, 10, 13, 18, 23, 27, 28]. The “ground glass” appearance results from the sclerotic dysplastic bone-like material and the radiolucent fibrous component of the lesion [29, 30]. A highly characteristic radiographic feature is bucco-lingual expansion with thinning of the cortical plate [3, 17, 23, 30–32]. This is best recognized on CT scans on bone windows. [3, 31]
Radiographically osteitis fibrosa also appears as a ground glass expansile lesion. However, osteitis fibrosa has an older adult onset compared to FD. In addition, abnormal levels of PTH, calcium, phosphorus, and defective vitamin D metabolism help to distinguish this entity from FD [33].
In addition to classic “ground glass” appearance, various other radiographic presentations of FD are mentioned in the literature, namely radiolucent, sclerotic, diffusely sclerotic, mixed radiolucent and radiopaque, smoky, cloudy, peau d’orange, finger print, chalky, pagetoid, and cotton wool [3–6, 8, 10, 11, 16, 18, 23, 27, 30, 31, 34, 35]. Very few patients in our study presented with mixed RO/RL, soap bubble radiolucency, mottled radiopacity, or multilocular RL with corticated margins.
While some consider upward displacement of the inferior mandibular canal a feature suggestive of and unique to fibrous dysplasia [36], others have reported displacement of the canal in all four directions [3, 16, 27, 31, 32]. The inferior alveolar canal displacement in various directions may depend on the location of the lesional epicenter in relation to the canal [31]. In our study, only one of the available radiographs displayed displacement of inferior alveolar canal and that was in a downward direction.
The loss of lamina dura of all the teeth within the lesion is another characteristic feature of FD, which can be used to confirm the radiological diagnosis of this condition [1, 27, 31, 32, 36]. In our study the loss of lamina dura was evident in all of the cases for which radiographs were available and where dentulous areas were involved. Root resorption is uncommon in fibrous dysplasia [3, 7, 10, 13, 18] and was also a rare finding in our study with only 3 of 22 cases demonstrating this feature.
Many of these radiographic features are not pathognomonic for gnathic fibrous dysplasia and may be seen in conditions like Paget’s disease of bone and cemento-ossifying fibroma. Hence, correlation of clinical features with microscopic presentation and imaging studies is critical for a definitive diagnosis as well as for evaluation of the extent of the lesion [3, 4, 6].
Clinical features such as the duration, site, and size of the lesion must be considered as well. Earlier smaller lesions may not induce drastic changes. In fact, many early lesions may be left undiagnosed due to lack of symptoms and/or difficulty in diagnosing early lesions on the radiographs.
Unfortunately, in some cases, biopsy specimens may not contain representative lesional tissue due to superficial sampling, suboptimal choice for biopsy location, and insufficient lesional tissue. Also, specimens taken by curettage result in fragmented specimens making the distinction between FD and other fibro-osseous lesions difficult if not impossible [3, 4]. In such cases, definitive diagnosis of bone lesions relies heavily on the imaging studies, which allow for the evaluation of the nature and the extent of the lesion, its behavior and effect on the adjacent anatomical structures. Likewise, when imaging studies are not conclusive, correlation with histopathological findings is necessary [3, 21, 37].
Conventional radiographs may not be the best mode for the evaluation of monostotic FD lesions due to poorly-defined margins, which may not draw clinician’s attention to the presence of the FD lesion. In one study, only 2% of monostotic FD were discovered incidentally [17]. CT scans may aid in better evaluation of the expansion, cortical borders, and lesion’s internal structure [16, 21, 23, 31, 38, 39]. CT features of FD need to be more widely reported. It must be noted that radiographic patterns observed in CT imaging and on conventional radiographs bear similarities. FD on the CT appears as homogenous gray hazy density described as ground-glass. CT presentation of FD is grouped into three categories: predominantly lytic or cyst-like (11–22%), mixed (40–55%), and predominantly sclerotic (34–38%) [40].
Classic Histopathologic Presentation and Histopathologic Variations
Fibrous dysplasia has a variety of histologic patterns, which may potentially create difficulty at arriving at the definitive diagnosis especially in the absence of imaging studies and a thorough clinical history. Greater awareness of differing patterns is necessary to aid in proper diagnosis. FD is described as immature, delicate, and curvilinear bony trabeculae with variable degree of mineralization and displaying classic “Chinese character” pattern with minimal or no osteoblastic rimming within a vascularized fibrous stroma of variable cellularity [3, 4, 6–8, 10, 11, 13].
During the early phase, a prominent osteogenesis may be evidenced by the presence of thin anastomosing woven bone trabeculae rimmed by osteoblasts. The stroma appears hypercellular and active and lacks pleomorphism. As the disease progresses, woven trabeculae become thicker and begin to assume the characteristic “Chinese letter” shape. The fibrous stroma remains hypercellular. Examination of the lesional specimen under polarized light reveals woven bone displaying haphazard arrangement of immature matrix [5, 11].
During later stages, the replacement of woven bone by lamellar bone becomes apparent and resting and reversal lines may result from the extensive remodeling [5].
Histopathologic differential diagnoses for FD include conventional and juvenile ossifying fibromas. FD exhibits a rather monotonous appearance with a constant presence of bone and fibrous tissue throughout the entire lesion; cementoid material is virtually absent. In contrast, ossifying fibroma is characterized by a high variability in stromal cellularity and types of mineralized material [37]. Juvenile ossifying fibroma shows a highly cellular fibroblastic stroma and numerous psammoma bodies (psammomatoid type) or “paint-brush strokes” trabeculae and more mature woven boney trabeculae (trabecular type) [6].
Riminnuci et al. described other patterns of FD such as Pagetoid and hypercellular. The Pagetoid pattern is characterized by thick, interconnected bony trabeculae with resting and reversal lines arranged in the mosaic-like pattern which histologically resembles Paget disease [41]. The hypercellular pattern is characterized by bony trabeculae arranged in a parallel manner and exhibiting osteoblastic rimming and numerous osteocytes [41].
Secondary changes in FD include formation of aneurysmal bone cyst, peritrabecular clefting, osteoblastic rimming, lamellar bone pattern, resting and reversal lines, and cementoid bodies. A biopsy taken from an area of secondary change may potentially lead to misdiagnosis [7, 8, 10, 11].
While peritrabecular clefting as a microscopic finding was mentioned to some extent in the literature, it was not considered as an important histopathologic feature [4, 5, 42]. In a multicenter study using image analyzer, 37 randomly selected samples of fibrous dysplasia were compared with 37 randomly samples of central ossifying fibroma. Peritrabecular clefting was observed in 32 (86.5%) cases of fibrous dysplasia versus none in central ossifying fibroma cases [9]. Soyele et al. analyzed patterns of fibro-osseous lesions and reported a “phenomenon of artificial retraction” in both FD (16/24) and ossifying fibromas (22/40) [12]. In our study, peritrabecular clefting was observed in all but three specimens, leading us to conclude that this is an important diagnostic feature.
Lack of osteoblastic rimming is an important feature of classic FD as reported in the literature [1, 3, 18, 34]. However, the presence of osteoblastic rimming to some extent is noted in more recent literature and this feature was observed in 42.5% of our specimens [4–6, 10, 11, 22, 42, 43]. While osteoblastic rimming has been reported around thin osteoid trabeculae in early stages of FD, similar rimming has also been reported around more mature lesional tissue [1, 5, 6]. Regardless of the stage, the presence of osteoblastic rimming to some degree may be present in FD. Hence, based on more recent literature reports and our findings we concluded that the presence of osteoblastic rimming should not exclude FD from diagnostic consideration.
Mature lamellar bone has been reported in long standing gnathic fibrous dysplasia and lesions tend to exhibit maturation into lamellar bone over time [1–8, 10, 18, 22, 42]. Moreover, the lamellar trabeculae exhibit a more parallel arrangement [3, 4, 6, 7, 42]. Such replacement of irregular and delicate trabeculae with elongated and parallel bony trabeculae is not seen in long bones of the skeleton [1–4, 7, 18, 42, 44]. Lesional specimens from older patients may show more lamellar bone compared to the specimens from younger patients [1, 6–8, 22]. While 52.5% of our specimens did not exhibit lamellar bone, one-fourth were composed mainly of lamellar bone. We attribute this finding to an older average age of the patients in this study.
Resting and reversal lines may be observed in lesions with extensive bone remodeling [5]. Over half of the specimens in our study lacked resting and reversal lines. Approximately 40% had focal to moderate presence of this feature. We therefore concluded that resting and reversal lines may not be a prominent feature in FD.
Cementoid bodies are concentrically formed laminations. It is a feature that is most commonly seen in cemento-osseous dysplasia (COD) as well as cemento-ossifiying fibroma. However, cementoid bodies may be seen to some extent in fibrous dysplasia [3, 4, 6, 11, 12, 22, 42], but are generally minimal and not considered a characteristic feature. Most of our specimens were lacking cementoid bodies, which is consistent with existing literature.
Evidence of the lesional bone fusing with the unaffected bone is very helpful in diagnosing FD [3, 4]. Such transition of lesional bone to normal bone explains the ill-defined borders on imaging studies. In our study, evidence of the lesion transitioning to normal bone was present in 32.5% of the specimens.
Management and Disease Progression
Craniofacial and gnathic FD lesions are subtyped into stabilized or quiescent, non-aggressive, or aggressive. Stabilized lesions exhibit no growth, non-aggressive lesions show slow growth, and aggressive lesions grow rapidly and may be associated with pain, paresthesia, malignant transformation [38]. Stabilization of FD usually occurs with skeletal maturation [3, 7, 8]. Therefore, clinicians are typically advised to opt for conservative management such as observation until after puberty [3, 6, 7].
Conservative surgical treatment, such as debulking, contouring and shaving may be performed for extensive lesions. However regrowth, especially in younger patients, may occur [3, 7]. Lesions that are refractory to debulking, small monostotic lesions, and aggressive lesions may require complete surgical removal [38]. Biopsy is recommended for aggressive lesions prior to surgical intervention to rule out secondary lesions such as aneurysmal bone cyst (ABC), osteomyelitis, or malignancy [38]. In such lesions, treatment is based on the associated lesion and may range from curettage (e.g., ABC) with contouring of FD to resection with adequate margins (e.g., malignancies) [38]. While bisphosphonates or denosumab may help relieve pain and slow lesional growth, more studies are needed to evaluate the long-term effects of such treatments [21, 38, 45–47]. Radiation therapy is contraindicated due to increased risk of sarcomatous transformation [42, 48].
Malignant transformation of FD is rare and comprises less than 1% of all cases [6, 7, 11, 21, 39, 49, 50]. Most of the malignancies develop in patients who underwent radiation therapy [3, 7, 11, 21, 42]. Malignancies arising from FD include osteosarcoma (~ 70%), fibrosarcoma (~ 20%), chondrosarcoma (~ 10%), and malignant fibrous histiocytoma (~ 4%) [3, 47]. Low-grade osteosarcomas may be more difficult to distinguish from fibrous dysplasia. Features such as well-formed osteoid, few mitoses and absence of marked atypia may present pathologist with diagnostic dilemma when distinguishing between low-grade osteosarcoma and FD [4, 51]. A study proposed the use of murine double-minute type 2 (MDM2) and cyclin-dependent kinase 4 (CDK4) markers in such cases to aid in proper diagnosis. In their study they demonstrated that malignancies express MDM2 and CDK4 markers, while benign fibrous and fibro-osseous lesions do not [51, 52]. Changes in clinical or radiographic presentation over time in gnathic FD patients should prompt further investigation to rule out malignant transformation [7, 39, 49]. Hence, long-term clinical follow-up is imperative.
Conclusion
A conclusive diagnosis of FD using a single diagnostic modality may prove to be a significant challenge given the myriad of clinical, radiographic, and histologic presentations. Clinicians and pathologists therefore, should refrain from making a diagnosis of FD or excluding FD without careful review of complete information including a CT, clinical history and adequate biopsy specimen, preferably a core biopsy submission if clinically possible and indicated.
Compliance with Ethical Standards
Conflict of interest
All authors declare no conflicts of interest.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was not required for this study under an approved waiver by the institutional review board at University of Florida.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.El-Mofty SK, Nelson B, Toyosawa S. Fibrous Dysplasia. In: WHO classification of head and neck tumors, 4th Edition. Lyon: IARC; 2017. pp. 253–254.
- 2.Hall G. Fibro-osseous lesions of the head and neck. Diagn Histopathol. 2012;18(4):149–158. [Google Scholar]
- 3.Brannon RB, Fowler CB. Benign fibro-osseous lesions: a review of current concepts. Adv Anat Pathol. 2001;8(3):126–143. doi: 10.1097/00125480-200105000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Speight PM, Carlos R. Maxillofacial fibro-osseous lesions. Curr Diagn Pathol. 2006;12(1):1–10. [Google Scholar]
- 5.Eversole R, Su L, ElMofty S. Benign fibro-osseous lesions of the craniofacial complex a review. Head Neck Pathol. 2008;2(3):177–202. doi: 10.1007/s12105-008-0057-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nwizu NN, Aguirre A, Chen F. Diagnostic challenges of benign fibro-osseous lesions and psammomatous meningiomas of the craniofacial region: a comparative review of their clinico-pathological features. N Am J Med Sci. 2010;3(1):17–23. [Google Scholar]
- 7.Waldron CA. Fibro-osseous lesions of the jaws. J Oral Maxillofac Surg. 1993;51(8):828–835. doi: 10.1016/s0278-2391(10)80097-7. [DOI] [PubMed] [Google Scholar]
- 8.Regezi JA. Odontogenic cysts, odontogenic tumors, fibroosseous, and giant cell lesions of the jaws. Mod Pathol. 2002;15(3):331–341. doi: 10.1038/modpathol.3880527. [DOI] [PubMed] [Google Scholar]
- 9.Prado Ribeiro AC, Carlos R, Speight PM, Hunter KD, Santos-Silva AR, de Almeida OP, Vargas PA. Peritrabecular clefting in fibrous dysplasia of the jaws: an important histopathologic feature for differentiating fibrous dysplasia from central ossifying fibroma. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012;114(4):503–508. doi: 10.1016/j.oooo.2012.06.014. [DOI] [PubMed] [Google Scholar]
- 10.Prabhu S, Sharanya S, Naik PM, Reddy A, Patil V, Pandey S, et al. Fibro-osseous lesions of the oral and maxillo-facial region: retrospective analysis for 20 years. J Oral Maxillofac Pathol. 2013;17(1):36. doi: 10.4103/0973-029X.110707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dorfman HD. New knowledge of fibro-osseous lesions of bone. Int J Surg Pathol. 2010;18(3 Suppl):62–65. doi: 10.1177/1066896910369924. [DOI] [PubMed] [Google Scholar]
- 12.Soyele O, Aborisade A, Olatunji S, Adesina O, Njokanma A. Patterns of fibro-osseous lesions of the oral and maxillofacial region seen in a tertiary hospital at Ile-Ife, Nigeria. Afr J Oral Maxillofac Pathol Med. 2018;4(1):16–27. [Google Scholar]
- 13.Toyosawa S, Yuki M, Kishino M, Ogawa Y, Ueda T, Murakami S, et al. Ossifying fibroma vs fibrous dysplasia of the jaw: molecular and immunological characterization. Mod Pathol. 2007;20(3):389. doi: 10.1038/modpathol.3800753. [DOI] [PubMed] [Google Scholar]
- 14.Marie PJ, de Pollak C, Chanson P, Lomri A. Increased proliferation of osteoblastic cells expressing the activating Gs alpha mutation in monostotic and polyostotic fibrous dysplasia. Am J Pathol. 1997;150(3):1059. [PMC free article] [PubMed] [Google Scholar]
- 15.Netto JDNS, Cerri JM, Miranda ÁMMA, Pires FR. Benign fibro-osseous lesions: clinicopathologic features from 143 cases diagnosed in an oral diagnosis setting. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013;115(5):e56–e65. doi: 10.1016/j.oooo.2012.05.022. [DOI] [PubMed] [Google Scholar]
- 16.MacDonald-Jankowski DS. Fibro-osseous lesions of the face and jaws. Clin Radiol. 2004;59(1):11–25. doi: 10.1016/j.crad.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 17.MacDonald DS. Maxillofacial fibro-osseous lesions. Clin Radiol. 2015;70:25–35. doi: 10.1016/j.crad.2014.06.022. [DOI] [PubMed] [Google Scholar]
- 18.Alsharif MJ, Sun ZJ, Chen XM, Wang SP, Zhao YF. Benign fibro-osseous lesions of the jaws: a study of 127 Chinese patients and review of the literature. Int J Surg Pathol. 2009;17(2):122–134. doi: 10.1177/1066896908318744. [DOI] [PubMed] [Google Scholar]
- 19.Collins MT, Singer FR, Eugster E. McCune–Albright syndrome and the extraskeletal manifestations of fibrous dysplasia. Orphanet J Rare Dis. 2012;7(1):S4. doi: 10.1186/1750-1172-7-S1-S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Akintoye SO, Boyce AM, Collins MT. Dental perspectives in fibrous dysplasia and McCune–Albright syndrome. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013;116(3):e149–e155. doi: 10.1016/j.oooo.2013.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.DiCaprio MR, Enneking WF. Fibrous dysplasia: pathophysiology, evaluation, and treatment. JBJS. 2005;87(8):1848–1864. doi: 10.2106/JBJS.D.02942. [DOI] [PubMed] [Google Scholar]
- 22.Phattarataratip E, Pholjaroen C, Tiranon P. A clinicopathologic analysis of 207 cases of benign fibro-osseous lesions of the jaws. Int J Surg Pathol. 2014;22(4):326–333. doi: 10.1177/1066896913511985. [DOI] [PubMed] [Google Scholar]
- 23.MacDonald-Jankowski D. Fibrous dysplasia: a systematic review. Dentomaxillofac Radiol. 2009;38(4):196–215. doi: 10.1259/dmfr/16645318. [DOI] [PubMed] [Google Scholar]
- 24.Worawongvasu R, Songkampol K. Fibro-osseous lesions of the jaws: an analysis of 122 cases in Thailand. J Oral Pathol Med. 2010;39(9):703–708. doi: 10.1111/j.1600-0714.2010.00932.x. [DOI] [PubMed] [Google Scholar]
- 25.El-Mofty SK. Familial gigantiform cementoma. In: WHO classification of head and neck tumors, 4th Edition. Lyon: IARC; 2017. p. 253.
- 26.Smith BJ, Eveson JW. Paget’s disease of bone with particular reference to dentistry. J Oral Pathol Med. 1981;10(4):233–247. doi: 10.1111/j.1600-0714.1981.tb01270.x. [DOI] [PubMed] [Google Scholar]
- 27.Nityasri V, Haris PS, Bose T, Balan A. Fibrous dysplasia—a 13-year retrospective radiographic analysis in a south Indian population. Dentomaxillofac Radiol. 2011;40(5):282–289. doi: 10.1259/dmfr/32556437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ahmad M, Gaalaas L. Fibroosseous and other lesions of bone in the jaws. Radiol Clin N Am. 2017;56:91–104. doi: 10.1016/j.rcl.2017.08.007. [DOI] [PubMed] [Google Scholar]
- 29.Lisle DA, Monsour PAJ, Maskiell CD. Imaging of craniofacial fibrous dysplasia. J Med Imaging Radiat Oncol. 2008;52(4):325–332. doi: 10.1111/j.1440-1673.2008.01963.x. [DOI] [PubMed] [Google Scholar]
- 30.Hanifi B, Samil KS, Yasar C, Cengiz C, Ercan A, Ramazan D. Craniofacial fibrous dysplasia. Clin Imaging. 2013;37(6):1109–1115. doi: 10.1016/j.clinimag.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 31.Sontakke SA, Karjodkar FR, Umarji HR. Computed tomographic features of fibrous dysplasia of maxillofacial region. Imaging Sci Dent. 2011;41(1):23–28. doi: 10.5624/isd.2011.41.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.MacDonald-Jankowski DS, Li TK. Fibrous dysplasia in a Hong Kong community: the clinical and radiological features and outcomes of treatment. Dentomaxillofac Radiol. 2009;38(2):63–72. doi: 10.1259/dmfr/56740531. [DOI] [PubMed] [Google Scholar]
- 33.Lerman MA, Do C, Gunaratnam L, Kulkarni C, Tucker K, Woo SB. Localized mandibular enlargement in end-stage renal disease: two case reports and a review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012;113(3):384–390. doi: 10.1016/j.tripleo.2011.04.039. [DOI] [PubMed] [Google Scholar]
- 34.Moshy J, Dimba E, Ocholla T, Chindia M. Characteristic radiological and histological patterns of fibrous dysplasia and ossifying fibroma of the jaws at University of Nairobi Dental Teaching Hospital. Surg Sci. 2012;3(04):189. [Google Scholar]
- 35.Deshpande A, Naidu GS, Dara BGB, Gupta M. Craniofacial fibrous dysplasia: a summary of findings with radiological emphasis. J Indian Acad Oral Med Radiol. 2016;28(4):403. [Google Scholar]
- 36.Petrikowski CG, Pharoah MJ, Lee L, Grace MG. Radiographic differentiation of osteogenic sarcoma, osteomyelitis, and fibrous dysplasia of the jaws. Oral Surg Oral Med Oral Pathol Oral Radiol. 1995;80(6):744–750. doi: 10.1016/s1079-2104(05)80260-4. [DOI] [PubMed] [Google Scholar]
- 37.Slootweg PJ, Müller H. Differential diagnosis of fibro-osseous jaw lesions: a histological investigation on 30 cases. J Craniomaxillofac Surg. 1990;18(5):210–214. doi: 10.1016/s1010-5182(05)80413-5. [DOI] [PubMed] [Google Scholar]
- 38.Lee JS, FitzGibbon EJ, Chen YR, Kim HJ, Lustig LR, Akintoye SO, Collins MT, Kaban LB. Clinical guidelines for the management of craniofacial fibrous dysplasia. Orphanet J Rare Dis. 2012;7(Suppl):1–19. doi: 10.1186/1750-1172-7-S1-S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reis C, Genden EM, Bederson JB, Som PM. A rare spontaneous osteosarcoma of the calvarium in a patient with long-standing fibrous dysplasia: CT and MR findings. Br J Radiol. 2008;81(962):e31–e34. doi: 10.1259/bjr/19620568. [DOI] [PubMed] [Google Scholar]
- 40.Chen YR, Wong FH, Hsueh C, Lo LJ. Computed tomography characteristics of non-syndromic craniofacial fibrous dysplasia. Chang Gung Med J. 2002;25(1):1–8. [PubMed] [Google Scholar]
- 41.Riminucci M, Liu B, Corsi A, Shenker A, Spiegel AM, Robey PG, Bianco P. The histopathology of fibrous dysplasia of bone in patients with activating mutations of the Gsα gene: site-specific patterns and recurrent histological hallmarks. J Pathol. 1999;187(2):249–258. doi: 10.1002/(SICI)1096-9896(199901)187:2<249::AID-PATH222>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 42.Alawi F. Benign fibro-osseous diseases of the maxillofacial bones: a review and differential diagnosis. Pathol Patterns Rev. 2002;118(Suppl 1):S50–S70. doi: 10.1309/NUXA-JUT9-HA09-WKMV. [DOI] [PubMed] [Google Scholar]
- 43.Shidham VB, Chavan A, Rao RN, Komorowski RA, Asma Z. Fatty metamorphosis and other patterns in fibrous dysplasia. BMC Musculoskelet Disord. 2003;4(1):20. doi: 10.1186/1471-2474-4-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Slootweg PJ. Lesions of the jaws. Histopathology. 2009;54(4):401–418. doi: 10.1111/j.1365-2559.2008.03097.x. [DOI] [PubMed] [Google Scholar]
- 45.Ricalde P, Horswell BB. Craniofacial fibrous dysplasia of the fronto-orbital region: a case series and literature review. J Oral Maxillofac Surg. 2001;59(2):157–167. doi: 10.1053/joms.2001.20487. [DOI] [PubMed] [Google Scholar]
- 46.Liens D, Delmas PD, Meunier PJ. Long-term effects of intravenous pamidronate in fibrous dysplasia of bone. Lancet. 1994;343(8903):953–954. doi: 10.1016/s0140-6736(94)90069-8. [DOI] [PubMed] [Google Scholar]
- 47.Chapurlat RD, Delmas PD, Liens D, Meunier PJ. Long-term effects of intravenous pamidronate in fibrous dysplasia of bone. J Bone Miner Res. 1997;12(10):1746–1752. doi: 10.1359/jbmr.1997.12.10.1746. [DOI] [PubMed] [Google Scholar]
- 48.El-Mofty SK. Fibro-osseous lesions of the craniofacial skeleton: an update. Head Neck Pathol. 2014;8(4):432–444. doi: 10.1007/s12105-014-0590-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sadeghi SM, Hosseini SN. Spontaneous conversion of fibrous dysplasia into osteosarcoma. J Craniofac Surg. 2011;22(3):959–961. doi: 10.1097/SCS.0b013e31820fe2bd. [DOI] [PubMed] [Google Scholar]
- 50.Ruggieri P, Sim FH, Bond JR, Krishnan Unni K. Malignancies in fibrous dysplasia. Cancer. 1994;73(5):1411–1424. doi: 10.1002/1097-0142(19940301)73:5<1411::aid-cncr2820730516>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 51.Dujardin F, Binh MBN, Bouvier C, Gomez-Brouchet A, Larousserie F, De Muret A, Louis-Brennetot C, Aurias A, Coindre JM, Guillou L, Pedeutour F. MDM2 and CDK4 immunohistochemistry is a valuable tool in the differential diagnosis of low-grade osteosarcomas and other primary fibro-osseous lesions of the bone. Mod Pathol. 2011;24(5):624. doi: 10.1038/modpathol.2010.229. [DOI] [PubMed] [Google Scholar]
- 52.Yoshida A, Ushiku T, Motoi T, Shibata T, Beppu Y, Fukayama M, Tsuda H. Immunohistochemical analysis of MDM2 and CDK4 distinguishes low-grade osteosarcoma from benign mimics. Mod Pathol. 2010;23(9):1279. doi: 10.1038/modpathol.2010.124. [DOI] [PubMed] [Google Scholar]