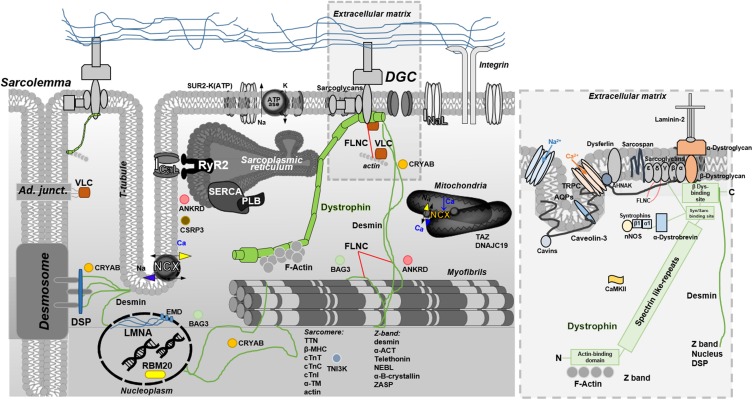
FIGURE 2.
Overview of full-length dystrophin in the context of other DCM-related proteins. The blow-up box, is a focus on full-length dystrophin structure and interactions. Full-length dystrophin is a large rod-shaped protein with a molecular weight of 427 kDa composed by 4 structural domains. The amino (N)-terminal domain has homology with α-actinin and binds, in particular, the F-actin; the central rod-domain contains 24–25 spectrin-like repeats the cysteine-rich domain intereacting with syntrophin and sarcoglycans; the last carboxy (C)-terminal domain associates at the C-terminal with β-dystroglican and several other proteins to form a major protein complex referred to as the dystrophin glycoproteic complex (DGC) (Hoffman et al., 1987; Ervasti and Campbell, 1993). The DGC consists of α- and β-dystroglycan subunits, α-, β-, δ-, γ-, and ε-sarcoglycans, sarcospan, α- and β-syntrophins, α-dystrobrevin, and neuronal nitric oxide synthase (nNOS) (Mosqueira et al., 2013). DGC related-pathways include Ca2+ homeostasis and E-C coupling, mitochondrial function, motor protein interaction (sarcomere/Z-band), and gene expression. For instance, the acetylcholine receptor, the skeletal and cardiac isoforms of the voltage-gated sodium channels (Nav1.4 and Nav1.5, respectively), the L-type Ca2+ channel, aquaporin, and stretch-activated channel or transient receptor potential (TRP) cation channels (Shirokova and Niggli, 2013) are closely associated with the DGC via syntrophins. In the cardiac tissue, dystrophin is also associated to: Cavin-1 and Caveolin-3 (responsible for caveolae/T tubule formation), Ahnak1 (modulates L-type Ca2+ channel), CryAB (involved in cytoprotection and antiapoptosis), and Cipher (plays a role in muscle contraction maintaining the Z-line integrity and signaling). Dystrophin can be also target of phosphorylation by Calmodulin-dependent kinase II (CaMKII) that modulates the affinity for F-actin and syntrophin (Madhavan and Jarrett, 1994). Other short isoforms of dystrophin come from spliced variants and are expressed in several other tissues. In particular, the Dp71 is expressed in cardiac muscle and likely present in T-tubular membranes (Kaprielian and Severs, 2000; Kaprielian et al., 2000).