Fig. (1).
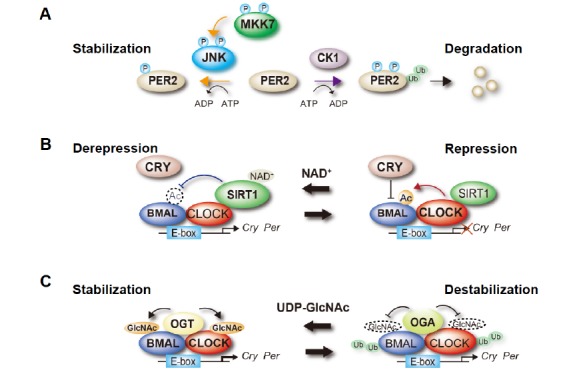
Functional regulation of clock proteins through post-translational modifications. (A) Phosphorylation-dependent control of PER2 stability. The activity of JNK is regulated via the phosphorylation of the tyrosine and threonine residues located in the kinase domain, which is catalyzed by MKK7. MKK7-mediated JNK activation induces phosphorylation of PER2 and increases its protein half-life by competing with the CKI-induced ubiquitination and the subsequent degradation of PER2. (B) Regulation of CLOCK:BMAL-mediated transcription by BMAL acetylation. CLOCK acetylates its heterodimeric partner BMAL. This CLOCK-mediated acetylation increases the interaction of the CLOCK:BMAL complex with CRY, facilitating repression of the CLOCK:BMAL complex’s activity. SIRT1 deacetylates BMAL, which cancels the CRY-dependent repression of CLOCK:BMAL-mediated transcription. (C) O-GlcNAcylation-dependent regulation of CLOCK:BMAL complex’s stability. Both CLOCK and BMAL1 are O-GlcNAcylated, which is catalyzed by OGT and reversed by OGA. This modification stabilizes both proteins by inhibiting their ubiquitination.
