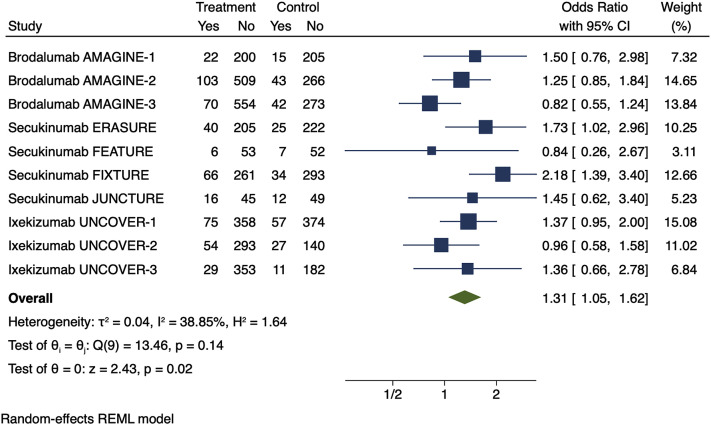
Fig 2.
Meta-estimate of respiratory tract infections (includes “upper respiratory tract infections,” “viral respiratory tract infections,” “influenza,” “influenza-like illness,” “sinusitis,” “pharyngitis,” “bronchitis,” “cough,” “nasopharyngitis,” “oropharyngeal pain,” and “pneumonia”) from clinicaltrials.gov in the phase 3 randomized control trials that were submitted for United States Food and Drug Administration approval. Doses used in this meta-estimate: secukinumab, 300 mg; brodalumab, 210 mg; and ixekizumab, 80 mg every 2 weeks, because these doses are indicated for moderate to severe psoriasis. The size of the square corresponds to the relative weight assigned in the pooled analysis, and the horizontal lines indicate the confidence interval (CI). The diamond denotes the overall effect size, and the lateral tips of the diamond indicate the associated CI. REML, Restricted maximum likelihood.