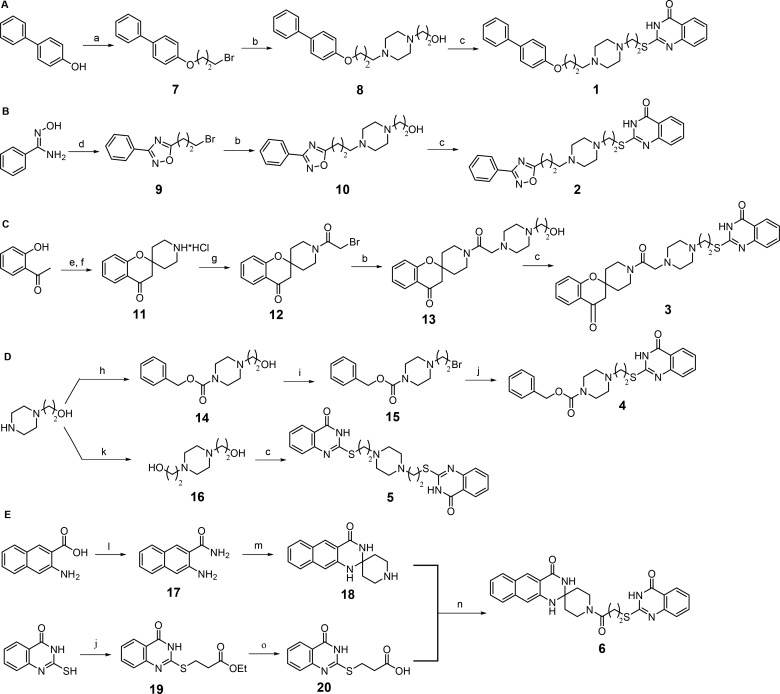
Scheme 1. Synthetic Routes to Compounds 1–6.
Reagents and conditions: (a) 1,3-dibromopropane, K2CO3, dry CH3CN, reflux; (b) 2-(piperazin-1-yl)ethan-1-ol, K2CO3, NaI, dry DMF, 60 °C; (c) 2-mercapto-4(3H)-quinazolinone, DIAD, 1 M trimethylphosphine in toluene, dry DMF, microwave, 40 °C; (d) (i) 4-bromobutanoyl chloride, TEA, dry DCM, rt, (ii) toluene, reflux; (e) tert-butyl 4-oxopiperidine-1-carboxylate, pyrrolidine, dry MeOH, reflux; (f) 4 M HCl in dioxane, dry THF; (g) bromoacetyl chloride, TEA, dry DCM, rt; (h) benzyloxycarbonyl chloride, TEA, dry THF, rt; (i) CBr4, triphenylphosphine, dry THF, rt; (j) 2-mercapto-4(3H)-quinazolinone, K2CO3, NaI, dry DMF, rt; (k) 2-bromoethan-1-ol, K2CO3, dry CH3CN, reflux; (l) NH3 aq., EDCI, HOBt, 4-methylmorpholine, dry THF, rt; (m) piperidin-4-one hydrochloride, concentrated H2SO4, CH3COOH, rt; (n) EDCI, HOBt, TEA, dry DMF, rt; (o) 2 M potassium hydroxide, EtOH, rt.