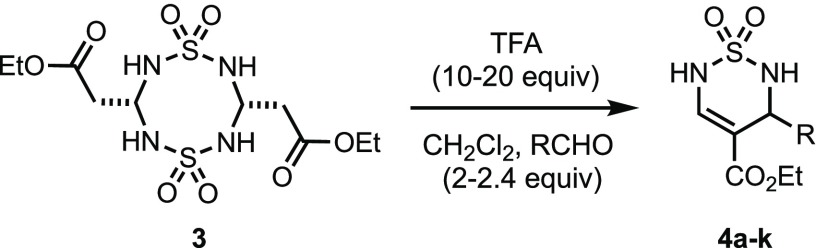
Table 2. Thiadiazine Formation with 3 and Various Aldehydes.
| Entry | R | 4a–j | Yielda |
|---|---|---|---|
| 1 | Ph | 4a | 66%b |
| 2 | Me | 4b | 59% |
| 3 | Et | 4c | 56% |
| 4 | 2,4-Cl2C6H3 | 4d | 70% |
| 5 | 4-NCC6H4 | 4e | 41% |
| 6 | 4-MeCO2C6H4 | 4f | 57% |
| 7 | 4-CF3C6H4 | 4g | 65% |
| 8 | 2-BrC6H4 | 4h | 45% |
| 9 | 4-AcOC6H4 | 4i | 48% |
| 10 | 3-MeOC6H4 | 4j | 62% |
| 11 | 3-thiophene | 4k | 30% |
Isolated yield after chromatography on SiO2.
Reaction was performed using TFA (2.5 equiv), HFIP, 35–40 °C, 17 h.