Figure 2. Recently identified human mutations in the AM-RAMP2-CLR signaling axis.
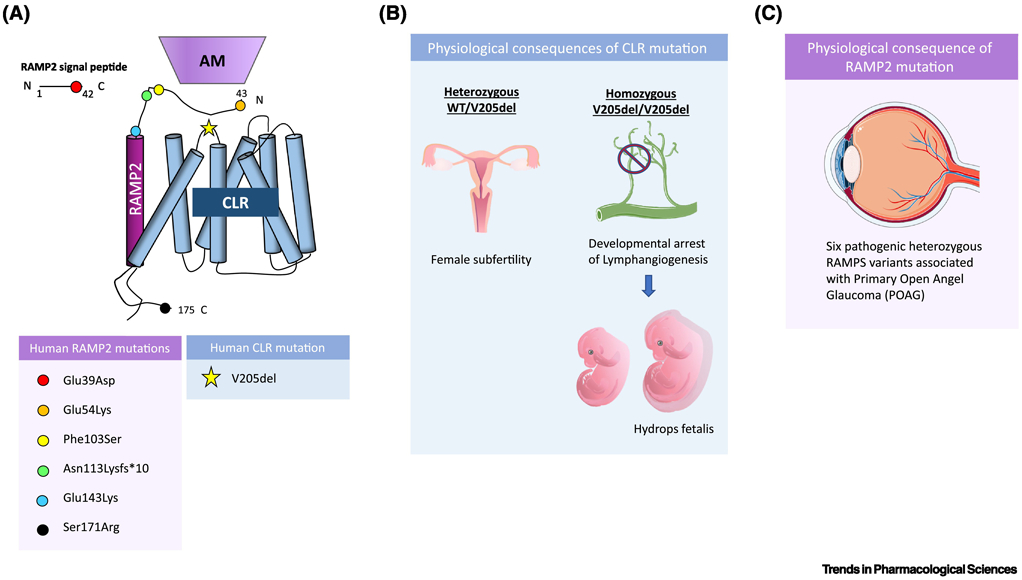
(A) As of 2019, in humans, a single pathologic mutation was identified in CLR, specifically on extracellular loop 1 of the receptor, and 6 heterozygous pathologic RAMP2 variants were identified, with mutations on the cleaved signaling peptide of RAMP2, and both the extracellular and cytosolic portion of the membrane-localized protein, with no mutations identified in the transmembrane domain. These mutations are spatially displayed on a schematic of CLR bound to RAMP2 and adrenomedullin, with each colored circle corresponding to one of the six identified RAMP2 variants and the star corresponding to the identified CLR mutation. (B) In humans, homozygous deletion of Valine 205 in CLR results in developmental arrest of lymphangiogenesis associated with lethal fluid accumulation, known as non-immune hydrops fetalis, while heterozygous carriers display female subfertility. Lymphatic deletion of CLR in mice results in similar phenotypes as seen in humans. (C) 6 pathologic RAMP2 variants were identified in an exome sequencing study of patients with primary open angle glaucoma (POAG) and each of the 6 variants were linked to functional impairments in AM-RAMP2-CLR signaling and deleterious effects on retinal ganglion nerve health.
