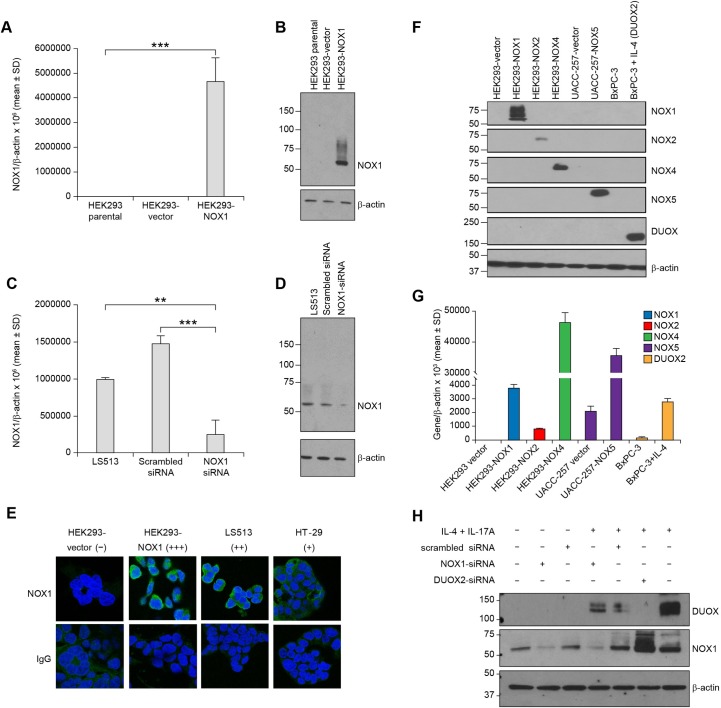
Fig 1. Characterization and validation of the novel NOX1 mouse monoclonal antibody.
(A, B) HEK293 cells were stably transfected with the pCMV-NOX1 plasmid or an empty vector and selected with G418. NOX1 overexpression was confirmed (A) at the mRNA level by RT-PCR (***p<0.001 vs. untransfected cells), and (B) at the protein level by Western blot analysis. (C, D) Transient knockdown of NOX1 expression in the colon cancer cell line LS513 with a scrambled control or a NOX1-specific siRNA. (C) A 4-fold decrease in NOX1 expression compared to parental (**p<0.01) and 6-fold decrease compared to cells transfected with scrambled siRNA (***p<0.001) was noted after 72 h at the mRNA level by RT-PCR. NOX1 mRNA level is given relative to β-actin. Data represent mean ± SD for at least 3 independent experiments. (D) Western blot analysis confirmed the NOX1 decrease at the protein level. (E) Immunodetection of NOX1 in HEK293-NOX1 and HEK293-vector control, LS513, and HT-29 cells by confocal microscopy. The cells were immunostained with NOX1 mouse mAb (green). Cell nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI; blue), and mouse anti-IgG was used as a negative control. Digital images were taken at 63X magnification. NOX1 protein expression is qualitatively labeled as -, no expression; +, relatively low expression; ++, relatively higher expression; +++, highest expression. (F) To demonstrate a lack of cross-reactivity of the NOX1 antibody with other NOX isoforms, protein levels were measured by Western blot analysis for HEK293 cells stably transfected with the pCMV-NOX1 plasmid, the Myc-DDK-tagged-NOX2 plasmid, the pCMV-MycDDK-HsNOX4 plasmid, or an empty vector; UACC-257-vector and UACC-257-NOX5 stable overexpressing clones; and BxPC-3 cells with or without IL-4 stimulation (25 ng/ml for 24 h). The antibodies used to detect the NOX isoforms are listed in the Materials and Methods section. (G) RNA levels of the NOX isoforms were measured by RT-PCR. (H) NOX1 detection in a real-world system, LS513 colon cancer cells, that express both NOX1 and DUOX2 following 24-h incubation with IL-4 plus IL-17A, and treated with either a NOX1- or DUOX2-specific small interfering RNA (siRNA), or a nonspecific scrambled siRNA.