Figure 5.
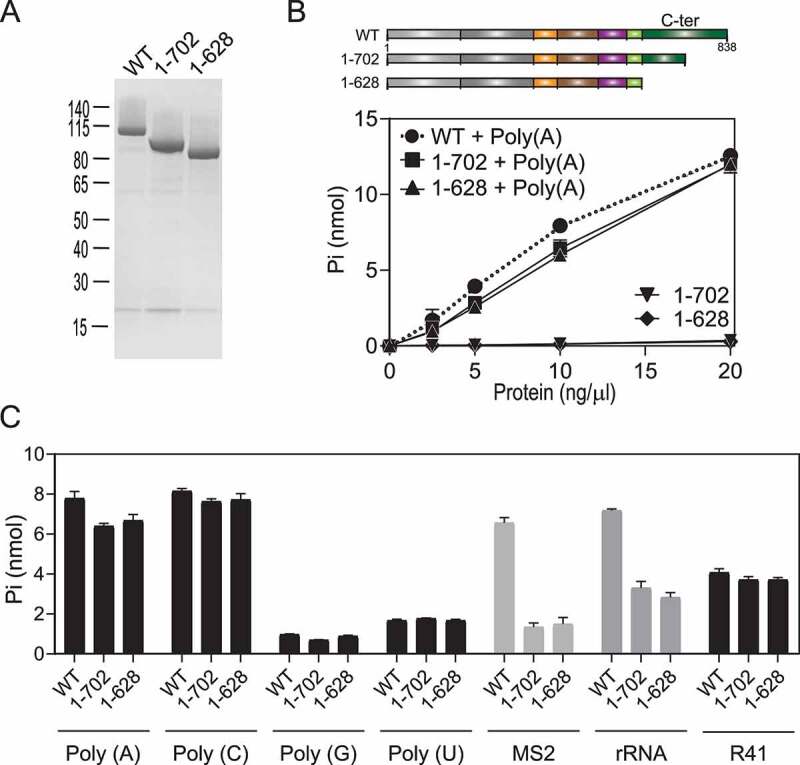
HrpB C-terminal deletion (1–702) and (1–628). (A) HrpB purification. Aliquots (2.5 µg) of the nickel-agarose preparations of wild-type (WT) HrpB, deletion mutant HrpB (1–702), and deletion mutant HrpB (1–628) were analysed by SDS-PAGE. A Coomassie Blue-stained gel is shown. The positions and sizes (in kDa) of marker proteins are indicated on the left. (B) ATPase activity. Reaction mixtures (15 µl) containing 50 mM Tris-HCl (pH 8.0), 1 mM DTT, 2 mM MgCl2, 1 mM [γ-32P] ATP, 250 ng/µl Poly(A) (or No RNA), and WT or mutant proteins as specified were incubated for 15 min at 37°C. Pi release was plotted as a function of input protein. (C) RNA specificity. Reaction mixtures (15 µl) containing 50 mM Tris-HCl (pH 8.0), 1 mM DTT, 2 mM MgCl2, 1 mM [γ-32P] ATP, and 250 ng/µl polyribonucleotide or RNA as specified (250 ng/µl of each polyribonucleotide, 80 ng/µl MS2, 100 ng/µl P. aeruginosa rRNA, and 5 µM R41) and 10 ng/µl of WT HrpB were incubated for 15 min at 37°C. The extends of Pi release are plotted. (B and C) Data are the average ± SEMs from three independent experiments.
