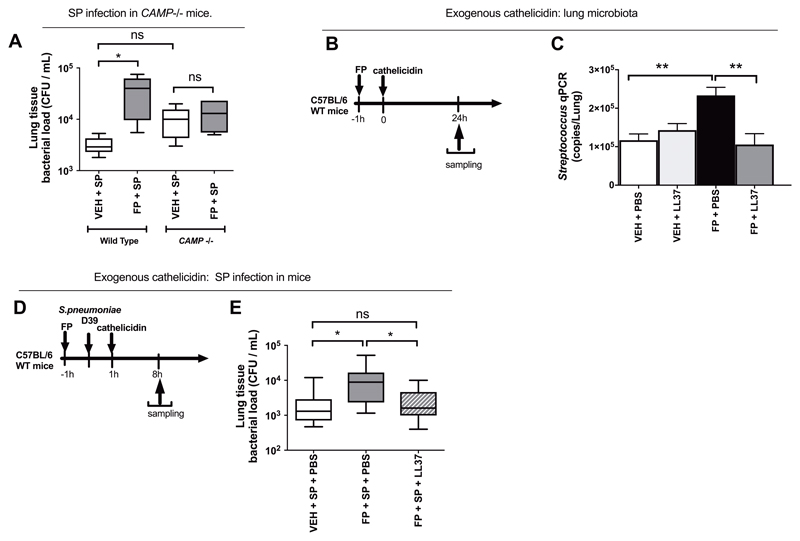
Figure 4. Impairment of pulmonary immunity by fluticasone propionate is dependent on cathelicidin.
(A) Wild type or CAMP -/- C57BL/6 mice were treated with 20ug fluticasone propionate or vehicle DMSO control and challenged intranasally with S. pneumoniae D39. Lung bacterial loads were measured by quantitative culture at 8h post-infection (B) Experimental outline. C57BL/6 mice were treated intranasally with 20ug fluticasone propionate or vehicle control and additionally with 10 ug recombinant LL-37. Lung tissue was harvested at 24h post-administration. (C) Lung Streptococcus was measured by qPCR (D) Experimental outline. C57BL/6 mice were treated intranasally with 20ug fluticasone propionate or vehicle DMSO control, challenged with S. pneumoniae D39 and additionally treated with 10 ug recombinant LL-37. Lung tissue was harvested at 8h post-administration. (E) Lung bacterial loads measured by quantitative culture. Data in (C) shown as mean (+/- S.E.M). Bacterial load data displayed as box and whisker plots showing median (line within box), IQR (box) and minimum to maximum (whiskers). Animal experiments comprise n=5-10 mice/group, representative of at least two independent experiments. Data analyzed by one-way ANOVA with Bonferroni post-test. n.s. non-significant *p<0.05 **p<0.01.